Evaluation of a recombinant measles virus expressing hepatitis C virus envelope proteins by infection of human PBL-NOD/Scid/Jak3null mouse
- PMID: 20299097
- PMCID: PMC7112578
- DOI: 10.1016/j.cimid.2010.02.006
Evaluation of a recombinant measles virus expressing hepatitis C virus envelope proteins by infection of human PBL-NOD/Scid/Jak3null mouse
Abstract
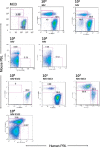
In this study, we infected NOD/Scid/Jak3null mice engrafted human peripheral blood leukocytes (hu-PBL-NOJ) with measles virus Edmonston B strain (MV-Edm) expressing hepatitis C virus (HCV) envelope proteins (rMV-E1E2) to evaluate the immunogenicity as a vaccine candidate. Although human leukocytes could be isolated from the spleen of mock-infected mice during the 2-weeks experiment, the proportion of engrafted human leukocytes in mice infected with MV (10(3)-10(5)pfu) or rMV-E1E2 (10(4)pfu) was decreased. Viral infection of the splenocytes was confirmed by the development of cytopathic effects (CPEs) in co-cultures of splenocytes and B95a cells and verified using RT-PCR. Finally, human antibodies against MV were more frequently observed than E2-specific antibodies in serum from mice infected with a low dose of virus (MV, 10(0)-10(1)pfu, and rMV-E1E2, 10(1)-10(2)pfu). These results showed the possibility of hu-PBL-NOJ mice for the evaluation of the immunogenicity of viral proteins.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
A Hepatitis C Virus DNA Vaccine Encoding a Secreted, Oligomerized Form of Envelope Proteins Is Highly Immunogenic and Elicits Neutralizing Antibodies in Vaccinated Mice.Front Immunol. 2019 May 24;10:1145. doi: 10.3389/fimmu.2019.01145. eCollection 2019. Front Immunol. 2019. PMID: 31178869 Free PMC article.
-
Native Folding of a Recombinant gpE1/gpE2 Heterodimer Vaccine Antigen from a Precursor Protein Fused with Fc IgG.J Virol. 2016 Dec 16;91(1):e01552-16. doi: 10.1128/JVI.01552-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795422 Free PMC article.
-
N-glycosylation-mutated HCV envelope glycoprotein complex enhances antigen-presenting activity and cellular and neutralizing antibody responses.Biochim Biophys Acta. 2016 Aug;1860(8):1764-75. doi: 10.1016/j.bbagen.2015.08.007. Epub 2015 Aug 14. Biochim Biophys Acta. 2016. PMID: 26278021
-
Resistance to human immunodeficiency virus 1 infection of SCID mice reconstituted with peripheral blood leukocytes from donors vaccinated with vaccinia gp160 and recombinant gp160.Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2443-7. doi: 10.1073/pnas.90.6.2443. Proc Natl Acad Sci U S A. 1993. PMID: 8460155 Free PMC article. Clinical Trial.
-
Prospects for prophylactic and therapeutic vaccines against the hepatitis C viruses.Immunol Rev. 2011 Jan;239(1):99-108. doi: 10.1111/j.1600-065X.2010.00977.x. Immunol Rev. 2011. PMID: 21198667 Review.
Cited by
-
A humanized mouse model identifies key amino acids for low immunogenicity of H7N9 vaccines.Sci Rep. 2017 Apr 28;7(1):1283. doi: 10.1038/s41598-017-01372-5. Sci Rep. 2017. PMID: 28455520 Free PMC article.
-
Versatility of live-attenuated measles viruses as platform technology for recombinant vaccines.NPJ Vaccines. 2022 Oct 15;7(1):119. doi: 10.1038/s41541-022-00543-4. NPJ Vaccines. 2022. PMID: 36243743 Free PMC article. Review.
-
Development of a duplex real-time RT-qPCR assay to monitor genome replication, gene expression and gene insert stability during in vivo replication of a prototype live attenuated canine distemper virus vector encoding SIV gag.J Virol Methods. 2015 Mar;213:26-37. doi: 10.1016/j.jviromet.2014.11.015. Epub 2014 Dec 5. J Virol Methods. 2015. PMID: 25486083 Free PMC article.
-
Development of Recombinant Measles Virus-Based Vaccines.Methods Mol Biol. 2017;1581:151-168. doi: 10.1007/978-1-4939-6869-5_9. Methods Mol Biol. 2017. PMID: 28374248 Free PMC article.
-
Vaccine platform recombinant measles virus.Virus Genes. 2017 Oct;53(5):733-740. doi: 10.1007/s11262-017-1486-3. Epub 2017 Jul 14. Virus Genes. 2017. PMID: 28710608 Free PMC article. Review.
References
-
- Di Bisceglie A.M., Carithers R.L., Jr., Gores G.J. Hepatocellular carcinoma. Hepatology. 1998;28(4):1161–1165. - PubMed
-
- Hayashi J. Hepatitis C virus and hepatocarcinogenesis. Intervirology. 1999;42(2–3):205–210. - PubMed
-
- Global surveillance and control of hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J Viral Hepat, 1999;6(1):35–47. - PubMed
-
- Zoulim F. Clinical consequences of hepatitis C virus infection. Rev Med Virol. 2003;13(1):57–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

