Awakening lineage potential by Ikaros-mediated transcriptional priming
- PMID: 20299195
- PMCID: PMC3869949
- DOI: 10.1016/j.coi.2010.02.011
Awakening lineage potential by Ikaros-mediated transcriptional priming
Abstract
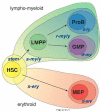
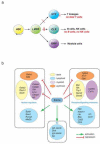
Bioinformatic studies on a revised hierarchy of hematopoietic progenitors have provided a genome-wide view of lineage-affiliated transcriptional programs directing early hematopoiesis. Unexpectedly, lymphoid, myeloid, and erythroid gene expression programs were primed with similar frequency at the multipotent progenitor stage indicating a stochastic nature to this process. Multilineage transcriptional priming is quickly resolved upon erythroid lineage restriction with both lymphoid and myeloid transcriptional programs rapidly extinguished. However, expression of lymphoid and myeloid factors remains active past nominal lymphoid and myeloid lineage restrictions, revealing a common genetic network utilized by both pathways. Priming and resolution of multilineage potential is dependent on the activity of the DNA binding factor Ikaros. Ikaros primes the lymphoid transcriptional program in the HSC and represses the stem cell and other disparate transcriptional programs downstream of the HSC. Loss of Ikaros removes the lymphoid leg of the immune system and may confer aberrant self-renewing properties to myeloid progenitors.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Genome-wide lineage-specific transcriptional networks underscore Ikaros-dependent lymphoid priming in hematopoietic stem cells.Immunity. 2009 Apr 17;30(4):493-507. doi: 10.1016/j.immuni.2009.01.014. Epub 2009 Apr 2. Immunity. 2009. PMID: 19345118 Free PMC article.
-
Ikaros fingers on lymphocyte differentiation.Int J Hematol. 2014 Sep;100(3):220-9. doi: 10.1007/s12185-014-1644-5. Epub 2014 Aug 2. Int J Hematol. 2014. PMID: 25085254 Free PMC article. Review.
-
An in vivo screen of noncoding loci reveals that Daedalus is a gatekeeper of an Ikaros-dependent checkpoint during haematopoiesis.Proc Natl Acad Sci U S A. 2021 Jan 19;118(3):e1918062118. doi: 10.1073/pnas.1918062118. Proc Natl Acad Sci U S A. 2021. PMID: 33446502 Free PMC article.
-
Early hematopoietic lineage restrictions directed by Ikaros.Nat Immunol. 2006 Apr;7(4):382-91. doi: 10.1038/ni1314. Epub 2006 Mar 5. Nat Immunol. 2006. PMID: 16518393 Free PMC article.
-
Lineage promiscuous expression of transcription factors in normal hematopoiesis.Int J Hematol. 2005 Jun;81(5):361-7. doi: 10.1532/ijh97.05003. Int J Hematol. 2005. PMID: 16158815 Review.
Cited by
-
Expression of Ik6 and Ik8 Isoforms and Their Association with Relapse and Death in Mexican Children with Acute Lymphoblastic Leukemia.PLoS One. 2015 Jul 1;10(7):e0130756. doi: 10.1371/journal.pone.0130756. eCollection 2015. PLoS One. 2015. PMID: 26131904 Free PMC article.
-
Alternative Splice Variants Modulates Dominant-Negative Function of Helios in T-Cell Leukemia.PLoS One. 2016 Sep 28;11(9):e0163328. doi: 10.1371/journal.pone.0163328. eCollection 2016. PLoS One. 2016. PMID: 27681508 Free PMC article.
-
Serine phosphorylation by SYK is critical for nuclear localization and transcription factor function of Ikaros.Proc Natl Acad Sci U S A. 2012 Oct 30;109(44):18072-7. doi: 10.1073/pnas.1209828109. Epub 2012 Oct 15. Proc Natl Acad Sci U S A. 2012. PMID: 23071339 Free PMC article.
-
Ikaros Inhibits Group 3 Innate Lymphoid Cell Development and Function by Suppressing the Aryl Hydrocarbon Receptor Pathway.Immunity. 2016 Jul 19;45(1):185-97. doi: 10.1016/j.immuni.2016.06.027. Immunity. 2016. PMID: 27438771 Free PMC article.
-
Ikaros interacts with P-TEFb and cooperates with GATA-1 to enhance transcription elongation.Nucleic Acids Res. 2011 May;39(9):3505-19. doi: 10.1093/nar/gkq1271. Epub 2011 Jan 17. Nucleic Acids Res. 2011. PMID: 21245044 Free PMC article.
References
-
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. [This paper shows that a combination of permissive and repressive histone modifications are associated on the promoter regions of transcription factors that are important for differentiation of the ES cells. This bivalent histone code is defined by presence of H3K27 and H3K4 indicating concomitant recruitment of dispsrate chromatin remodelers (PRC1/2 and Mll) Authors propose that bivalent histone domains repress developmental genes in ES cells while keeping them poised for activation.] - PubMed
-
- Su IH, Basavaraj A, Krutchinsky AN, Hobert O, Ullrich A, Chait BT, Tarakhovsky A. Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat Immunol. 2003;4:124–131. - PubMed
-
- McMahon KA, Hiew SY, Hadjur S, Veiga-Fernandes H, Menzel U, Price AJ, Kioussis D, Williams O, Brady HJ. Mll has a critical role in fetal and adult hematopoietic stem cell self-renewal. Cell Stem Cell. 2007;1:338–345. - PubMed
-
- Yoshida T, Zhang J, Hazan I, Naito T, Ng SY, Snippert HJ, Heller EJ, Lawton L, Williams CJ, Georgopoulos K. The role of the chromatin remodeler Mi-2beta in hematopoietic stem cell self-renewal and multi-lineage differentiation. Genes Dev. 2008;22:1174–1189. [This study demonstrates that the ATP-dependent chromatin remodeler Mi-2β, an Ikaros partner regulates two important properties of the HSC, self-renewal and multi-potency. Loss of Mi-2β, causes normally quiescent HSC to undergo aberrant cell divisions that result in their exhaustion. Loss of self-renewal correlates with loss of a significant fraction of stem cell specific genes. Mi-2β deficient HSC readily differentiate into the erythroid lineage but their differentiation in their myeloid is blocked downstream of the LMPP. Priming of the myeloid lineage program is severely affected in the HSC-LMPP compartment with dire consequences for LMPP's further differentiation into the myeloid pathway. This provides a diagramatically opposing scenario to that described for Ikaros mutant progenitors.] - PMC - PubMed
-
- Broske AM, Vockentanz L, Kharazi S, Huska MR, Mancini E, Scheller M, Kuhl C, Enns A, Prinz M, Jaenisch R, et al. DNA methylation protects hematopoietic stem cell multipotency from myeloerythroid restriction. Nat Genet. 2009;41:1207–1215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

