Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture
- PMID: 20299346
- PMCID: PMC2887065
- DOI: 10.1093/aob/mcq028
Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture
Abstract
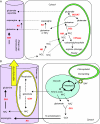
Background: Productive agriculture needs a large amount of expensive nitrogenous fertilizers. Improving nitrogen use efficiency (NUE) of crop plants is thus of key importance. NUE definitions differ depending on whether plants are cultivated to produce biomass or grain yields. However, for most plant species, NUE mainly depends on how plants extract inorganic nitrogen from the soil, assimilate nitrate and ammonium, and recycle organic nitrogen. Efforts have been made to study the genetic basis as well as the biochemical and enzymatic mechanisms involved in nitrogen uptake, assimilation, and remobilization in crops and model plants. The detection of the limiting factors that could be manipulated to increase NUE is the major goal of such research.
Scope: An overall examination of the physiological, metabolic, and genetic aspects of nitrogen uptake, assimilation and remobilization is presented in this review. The enzymes and regulatory processes manipulated to improve NUE components are presented. Results obtained from natural variation and quantitative trait loci studies are also discussed.
Conclusions: This review presents the complexity of NUE and supports the idea that the integration of the numerous data coming from transcriptome studies, functional genomics, quantitative genetics, ecophysiology and soil science into explanatory models of whole-plant behaviour will be promising.
Figures




References
-
- Andrews M, Lea PJ, Raven JA, Lindsay K. Can genetic manipulation of plant nitrogen assimilation enzymes result in increased crop yield and greater N-use efficiency? An assessment. Annals of Applied Biology. 2004;145:25–40.
-
- Baena-Gonzalez E, Rolland F, Thevelein JM, Sheen J. A central integrator of transcription networks in plant stress and energy signalling. Nature. 2007;448:938–942. - PubMed
-
- Bernard SM, Habash DZ. The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytologist. 2009;182:608–620. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

