Reactive oxygen species promote caspase-12 expression and tubular apoptosis in diabetic nephropathy
- PMID: 20299359
- PMCID: PMC2900966
- DOI: 10.1681/ASN.2009030242
Reactive oxygen species promote caspase-12 expression and tubular apoptosis in diabetic nephropathy
Abstract
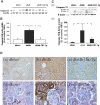
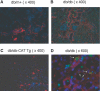
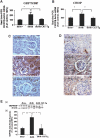
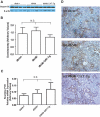
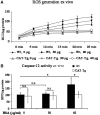
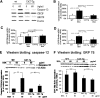
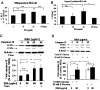
Apoptosis of tubular epithelial cells contributes to the tubular atrophy that accompanies diabetic nephropathy. Reactive oxygen species (ROS) promote tubular apoptosis, but the mechanisms by which this occurs are incompletely understood. Here, we sought proapoptotic genes that ROS differentially upregulate in renal proximal tubular cells of diabetic (db/db) mice. We performed microarray analysis using total RNA from freshly isolated renal proximal tubules of nondiabetic, diabetic, and diabetic transgenic mice overexpressing catalase in the proximal tubule (thereby attenuating ROS). We observed greater expression of caspase-12 in the proximal tubules of the diabetic mice compared with the nondiabetic and diabetic transgenic mice. Quantitative PCR and immunohistochemistry confirmed the enhanced expression of caspase-12, as well as members of the endoplasmic reticulum stress-induced apoptotic pathway. Ex vivo, albumin induced caspase-12 activity and expression (protein and mRNA) and mRNA expression of the CCAT/enhancer-binding protein homologous protein in freshly isolated wild-type proximal tubules but not in catalase-overexpressing proximal tubules. In vitro, albumin stimulated activity of both caspase-12 and caspase-3 as well as expression of caspase-12 and CCAT/enhancer-binding protein homologous protein in a human proximal tubule cell line (HK-2). The free radical scavenger tiron inhibited these effects. Furthermore, knockdown of caspase-12 with small interfering RNA reduced albumin-induced apoptosis in HK-2 cells. Taken together, these studies demonstrate that albuminuria may induce tubular apoptosis through generation of ROS and the subsequent expression and activation of endoplasmic reticulum stress genes in the diabetic kidney.
Figures



Comment in
-
Caspase-12 and diabetic nephropathy: from mice to men?J Am Soc Nephrol. 2010 Jun;21(6):886-8. doi: 10.1681/ASN.2010040394. Epub 2010 May 6. J Am Soc Nephrol. 2010. PMID: 20448021 No abstract available.
References
-
- Rabkin R: Diabetic nephropathy. Clin Cornerstone 5: 1–11, 2003. - PubMed
-
- Sarafidis PA, Stafylas PC, Kanaki AI, Lasaridis AN: Effects of renin-angiotensin system blockers on renal outcomes and all-cause mortality in patients with diabetic nephropathy: An updated meta-analysis. Am J Hypertens 21: 922–929, 2008. - PubMed
-
- Gordois A, Scuffham P, Shearer A, Oglesby A: The health care costs of diabetic nephropathy in the United States and the United Kingdom. J Diabetes Complications 18: 18–26, 2004. - PubMed
-
- Hovind P, Tarnow L, Parving HH: Remission and regression of diabetic nephropathy. Curr Hypertens Rep 6: 377–382, 2004. - PubMed
-
- Tarchini R, Bottini E, Botti P, Marseglia CD, Talassi E, Baraldi O, Lambertini D, Gaetti L, Bellomi A: Type 2 diabetic nephropathy: Clinical course and prevention proposals 2004 [in Italian]. G Ital Nefrol 22[Suppl 31]: S15–S19, 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

