Oxidants positively or negatively regulate nuclear factor kappaB in a context-dependent manner
- PMID: 20299457
- PMCID: PMC2871441
- DOI: 10.1074/jbc.M110.103259
Oxidants positively or negatively regulate nuclear factor kappaB in a context-dependent manner
Abstract
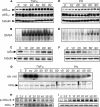
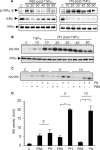
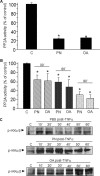
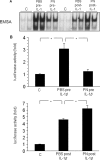
Redox-based mechanisms play critical roles in the regulation of multiple cellular functions. NF-kappaB, a master regulator of inflammation, is an inducible transcription factor generally considered to be redox-sensitive, but the modes of interactions between oxidant stress and NF-kappaB are incompletely defined. Here, we show that oxidants can either amplify or suppress NF-kappaB activation in vitro by interfering both with positive and negative signals in the NF-kappaB pathway. NF-kappaB activation was evaluated in lung A549 epithelial cells stimulated with tumor necrosis factor alpha (TNFalpha), either alone or in combination with various oxidant species, including hydrogen peroxide or peroxynitrite. Exposure to oxidants after TNFalpha stimulation produced a robust and long lasting hyperactivation of NF-kappaB by preventing resynthesis of the NF-kappaB inhibitor IkappaB, thereby abrogating the major negative feedback loop of NF-kappaB. This effect was related to continuous activation of inhibitor of kappaB kinase (IKK), due to persistent IKK phosphorylation consecutive to oxidant-mediated inactivation of protein phosphatase 2A. In contrast, exposure to oxidants before TNFalpha stimulation impaired IKK phosphorylation and activation, leading to complete prevention of NF-kappaB activation. Comparable effects were obtained when interleukin-1beta was used instead of TNFalpha as the NF-kappaB activator. This study demonstrates that the influence of oxidants on NF-kappaB is entirely context-dependent, and that the final outcome (activation versus inhibition) depends on a balanced inhibition of protein phosphatase 2A and IKK by oxidant species. Our findings provide a new conceptual framework to understand the role of oxidant stress during inflammatory processes.
Figures


Similar articles
-
PMC, a potent hydrophilic α-tocopherol derivative, inhibits NF-κB activation via PP2A but not IκBα-dependent signals in vascular smooth muscle cells.J Cell Mol Med. 2014 Jul;18(7):1278-89. doi: 10.1111/jcmm.12277. Epub 2014 Apr 13. J Cell Mol Med. 2014. PMID: 24725826 Free PMC article.
-
Hydrogen peroxide prolongs nuclear localization of NF-kappaB in activated cells by suppressing negative regulatory mechanisms.J Biol Chem. 2008 Jul 4;283(27):18582-90. doi: 10.1074/jbc.M801312200. Epub 2008 May 12. J Biol Chem. 2008. PMID: 18474597
-
Dual effect of oxidative stress on NF-kappakB activation in HeLa cells.Exp Mol Med. 2002 Nov 30;34(5):332-9. doi: 10.1038/emm.2002.47. Exp Mol Med. 2002. PMID: 12526096
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
-
Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity.Annu Rev Immunol. 2000;18:621-63. doi: 10.1146/annurev.immunol.18.1.621. Annu Rev Immunol. 2000. PMID: 10837071 Review.
Cited by
-
Topical hypochlorite ameliorates NF-κB-mediated skin diseases in mice.J Clin Invest. 2013 Dec;123(12):5361-70. doi: 10.1172/JCI70895. Epub 2013 Nov 15. J Clin Invest. 2013. PMID: 24231355 Free PMC article.
-
An Intimate Relationship between ROS and Insulin Signalling: Implications for Antioxidant Treatment of Fatty Liver Disease.Int J Cell Biol. 2014;2014:519153. doi: 10.1155/2014/519153. Epub 2014 Feb 12. Int J Cell Biol. 2014. PMID: 24672550 Free PMC article. Review.
-
Pyrrolidine dithiocarbamate administered during ex-vivo lung perfusion promotes rehabilitation of injured donor rat lungs obtained after prolonged warm ischemia.PLoS One. 2017 Mar 21;12(3):e0173916. doi: 10.1371/journal.pone.0173916. eCollection 2017. PLoS One. 2017. PMID: 28323904 Free PMC article.
-
Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death.Free Radic Biol Med. 2011 May 15;50(10):1368-81. doi: 10.1016/j.freeradbiomed.2011.02.021. Epub 2011 Mar 11. Free Radic Biol Med. 2011. PMID: 21362471 Free PMC article.
-
The role of ageing and oxidative stress in intervertebral disc degeneration.Front Mol Biosci. 2022 Nov 7;9:1052878. doi: 10.3389/fmolb.2022.1052878. eCollection 2022. Front Mol Biosci. 2022. PMID: 36419928 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

