A combined computational and functional approach identifies new residues involved in pH-dependent gating of ASIC1a
- PMID: 20299463
- PMCID: PMC2871499
- DOI: 10.1074/jbc.M109.092015
A combined computational and functional approach identifies new residues involved in pH-dependent gating of ASIC1a
Abstract
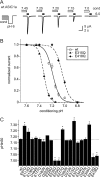
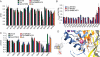
Acid-sensing ion channels (ASICs) are key receptors for extracellular protons. These neuronal nonvoltage-gated Na(+) channels are involved in learning, the expression of fear, neurodegeneration after ischemia, and pain sensation. We have applied a systematic approach to identify potential pH sensors in ASIC1a and to elucidate the mechanisms by which pH variations govern ASIC gating. We first calculated the pK(a) value of all extracellular His, Glu, and Asp residues using a Poisson-Boltzmann continuum approach, based on the ASIC three-dimensional structure, to identify candidate pH-sensing residues. The role of these residues was then assessed by site-directed mutagenesis and chemical modification, combined with functional analysis. The localization of putative pH-sensing residues suggests that pH changes control ASIC gating by protonation/deprotonation of many residues per subunit in different channel domains. Analysis of the function of residues in the palm domain close to the central vertical axis of the channel allowed for prediction of conformational changes of this region during gating. Our study provides a basis for the intrinsic ASIC pH dependence and describes an approach that can also be applied to the investigation of the mechanisms of the pH dependence of other proteins.
Figures

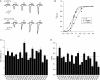

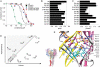
References
-
- Krishtal O. (2003) Trends Neurosci. 26, 477–483 - PubMed
-
- Wemmie J. A., Price M. P., Welsh M. J. (2006) Trends Neurosci. 29, 578–586 - PubMed
-
- Kellenberger S., Schild L. (2002) Physiol. Rev. 82, 735–767 - PubMed
-
- Sluka K. A., Price M. P., Breese N. M., Stucky C. L., Wemmie J. A., Welsh M. J. (2003) Pain 106, 229–239 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

