TBC1D1 regulates insulin- and contraction-induced glucose transport in mouse skeletal muscle
- PMID: 20299473
- PMCID: PMC2874696
- DOI: 10.2337/db09-1266
TBC1D1 regulates insulin- and contraction-induced glucose transport in mouse skeletal muscle
Abstract
Objective: TBC1D1 is a member of the TBC1 Rab-GTPase family of proteins and is highly expressed in skeletal muscle. Insulin and contraction increase TBC1D1 phosphorylation on phospho-Akt substrate motifs (PASs), but the function of TBC1D1 in muscle is not known. Genetic linkage analyses show a TBC1D1 R125W missense variant confers risk for severe obesity in humans. The objective of this study was to determine whether TBC1D1 regulates glucose transport in skeletal muscle.
Research design and methods: In vivo gene injection and electroporation were used to overexpress wild-type and several mutant TBC1D1 proteins in mouse tibialis anterior muscles, and glucose transport was measured in vivo.
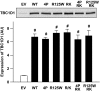
Results: Expression of the obesity-associated R125W mutant significantly decreased insulin-stimulated glucose transport in the absence of changes in TBC1D1 PAS phosphorylation. Simultaneous expression of an inactive Rab-GTPase (GAP) domain of TBC1D1 in the R125W mutant reversed this decrease in glucose transport caused by the R125W mutant. Surprisingly, expression of TBC1D1 mutated to Ala on four conserved Akt and/or AMP-activated protein kinase predicted phosphorylation sites (4P) had no effect on insulin-stimulated glucose transport. In contrast, expression of the TBC1D1 4P mutant decreased contraction-stimulated glucose transport, an effect prevented by concomitant disruption of TBC1D1 Rab-GAP activity. There was no effect of the R125W mutation on contraction-stimulated glucose transport.
Conclusions: TBC1D1 regulates both insulin- and contraction-stimulated glucose transport, and this occurs via distinct mechanisms. The R125W mutation of TBC1D1 impairs skeletal muscle glucose transport, which could be a mechanism for the obesity associated with this mutation.
Figures
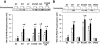
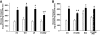
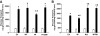
References
-
- Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ: Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 1998; 47: 1369–1373 - PubMed
-
- Goodyear LJ, Kahn BB: Exercise, glucose transport, and insulin sensitivity. Annu Rev Med 1998; 49: 235–261 - PubMed
-
- Zierath JR, Krook A, Wallberg-Henriksson H: Insulin action in skeletal muscle from patients with NIDDM. Mol Cell Biochem 1998; 182: 153–160 - PubMed
-
- Kennedy JW, Hirshman MF, Gervino EV, Ocel JV, Forse RA, Hoenig SJ, Aronson D, Goodyear LJ, Horton ES: Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes 1999; 48: 1192–1197 - PubMed
-
- Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ: Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes 2006; 55: 2067–2076 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

