Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation
- PMID: 20299509
- PMCID: PMC2879099
- DOI: 10.1182/blood-2009-10-245001
Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation
Abstract
Although red blood cell (RBC) transfusions can be lifesaving, they are not without risk. In critically ill patients, RBC transfusions are associated with increased morbidity and mortality, which may increase with prolonged RBC storage before transfusion. The mechanisms responsible remain unknown. We hypothesized that acute clearance of a subset of damaged, stored RBCs delivers large amounts of iron to the monocyte/macrophage system, inducing inflammation. To test this in a well-controlled setting, we used a murine RBC storage and transfusion model to show that the transfusion of stored RBCs, or washed stored RBCs, increases plasma nontransferrin bound iron (NTBI), produces acute tissue iron deposition, and initiates inflammation. In contrast, the transfusion of fresh RBCs, or the infusion of stored RBC-derived supernatant, ghosts, or stroma-free lysate, does not produce these effects. Furthermore, the insult induced by transfusion of stored RBC synergizes with subclinical endotoxinemia producing clinically overt signs and symptoms. The increased plasma NTBI also enhances bacterial growth in vitro. Taken together, these results suggest that, in a mouse model, the cellular component of leukoreduced, stored RBC units contributes to the harmful effects of RBC transfusion that occur after prolonged storage. Nonetheless, these findings must be confirmed by prospective human studies.
Figures
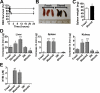
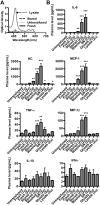

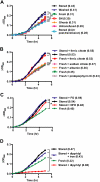
 ) or stored RBCs (n = 6; ■); *P < .01. (C) Circulating SAA1 protein levels in SAA1-luciferase reporter mice 24 hours after transfusion with fresh RBCs or stored RBCs (n = 6 per group); *P < .01. Results are combined from 2 separate experiments.
) or stored RBCs (n = 6; ■); *P < .01. (C) Circulating SAA1 protein levels in SAA1-luciferase reporter mice 24 hours after transfusion with fresh RBCs or stored RBCs (n = 6 per group); *P < .01. Results are combined from 2 separate experiments.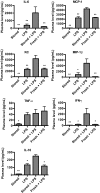
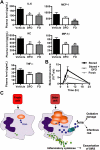
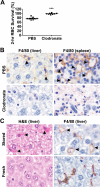
 ), the PBS vehicle control and stored RBCs (n = 3; ■), or 3 mg of DFO and stored RBCs (n = 6;
), the PBS vehicle control and stored RBCs (n = 3; ■), or 3 mg of DFO and stored RBCs (n = 6;  ); P = .095 at 4 and 6 hours after transfusion comparing vehicle-treated and DFO-treated mice. (C) Proposed mechanistic pathway (the “iron hypothesis”) explaining how transfusion of older stored RBCs may induce adverse effects in patients. Transfusion of stored, but not fresh, RBCs delivers an acute bolus of RBCs and RBC-derived iron to the monocyte/macrophage system resulting in oxidative stress and inflammatory cytokine secretion. Some of the macrophage-ingested iron is also released back into the circulation (ie, NTBI) where it can also cause oxidative damage and enhance bacterial proliferation. SIRS indicates systemic inflammatory response syndrome.
); P = .095 at 4 and 6 hours after transfusion comparing vehicle-treated and DFO-treated mice. (C) Proposed mechanistic pathway (the “iron hypothesis”) explaining how transfusion of older stored RBCs may induce adverse effects in patients. Transfusion of stored, but not fresh, RBCs delivers an acute bolus of RBCs and RBC-derived iron to the monocyte/macrophage system resulting in oxidative stress and inflammatory cytokine secretion. Some of the macrophage-ingested iron is also released back into the circulation (ie, NTBI) where it can also cause oxidative damage and enhance bacterial proliferation. SIRS indicates systemic inflammatory response syndrome.References
-
- Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48(6):1053–1060. - PubMed
-
- Whitaker B, Sullivan M. The 2005 Nationwide Blood Collection and Utilization Survey Report. Bethesda, MD: AABB; 2006.
-
- Luten M, Roerdinkholder-Stoelwinder B, Schaap NP, de Grip WJ, Bos HJ, Bosman GJ. Survival of red blood cells after transfusion: a comparison between red cells concentrates of different storage periods. Transfusion. 2008;48(7):1478–1485. - PubMed
-
- Zeiler T, Muller JT, Kretschmer V. Flow-cytometric determination of survival time and 24-hour recovery of transfused red blood cells. Transfus Med Hemother. 2003;30:14–19.
-
- Ozment CP, Turi JL. Iron overload following red blood cell transfusion and its impact on disease severity. Biochim Biophys Acta. 2009;1790(7):694–701. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

