E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin alphaLbeta2-mediated slow leukocyte rolling
- PMID: 20299514
- PMCID: PMC2913455
- DOI: 10.1182/blood-2009-12-259556
E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin alphaLbeta2-mediated slow leukocyte rolling
Abstract
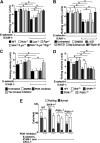
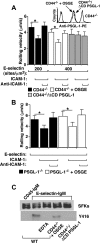
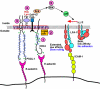
In inflamed venules, neutrophils rolling on E-selectin induce integrin alpha(L)beta(2)-dependent slow rolling on intercellular adhesion molecule-1 by activating Src family kinases (SFKs), DAP12 and Fc receptor-gamma (FcRgamma), spleen tyrosine kinase (Syk), and p38. E-selectin signaling cooperates with chemokine signaling to recruit neutrophils into tissues. Previous studies identified P-selectin glycoprotein ligand-1 (PSGL-1) as the essential E-selectin ligand and Fgr as the only SFK that initiate signaling to slow rolling. In contrast, we found that E-selectin engagement of PSGL-1 or CD44 triggered slow rolling through a common, lipid raft-dependent pathway that used the SFKs Hck and Lyn as well as Fgr. We identified the Tec kinase Bruton tyrosine kinase as a key signaling intermediate between Syk and p38. E-selectin engagement of PSGL-1 was dependent on its cytoplasmic domain to activate SFKs and slow rolling. Although recruiting phosphoinositide-3-kinase to the PSGL-1 cytoplasmic domain was reported to activate integrins, E-selectin-mediated slow rolling did not require phosphoinositide-3-kinase. Studies in mice confirmed the physiologic significance of these events for neutrophil slow rolling and recruitment during inflammation. Thus, E-selectin triggers common signals through distinct neutrophil glycoproteins to induce alpha(L)beta(2)-dependent slow rolling.
Figures




Comment in
-
Neutrophil CD44 rafts and rolls.Blood. 2010 Jul 22;116(3):314-5. doi: 10.1182/blood-2010-04-276410. Blood. 2010. PMID: 20651082 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

