Decreased phospho-Akt signaling in a mouse model of total parenteral nutrition: a potential mechanism for the development of intestinal mucosal atrophy
- PMID: 20299605
- PMCID: PMC3774331
- DOI: 10.1152/ajpgi.00030.2010
Decreased phospho-Akt signaling in a mouse model of total parenteral nutrition: a potential mechanism for the development of intestinal mucosal atrophy
Abstract
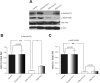
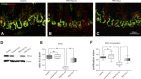
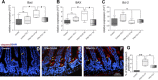
Total parenteral nutrition (TPN) leads to a decline in phosphatidylinositol 3-kinase (PI3K)/phospho-Akt (p-Akt) activity, affecting downstream signaling, reducing epithelial cell (EC) proliferation, and contributing to intestinal mucosal atrophy. We hypothesized that promoting Akt activity would prevent these changes. We used a novel Akt-activating peptide, TCL1 (a head-to-tail dimer of the Akt-binding domain of T-cell lymphoma-1), or an inactive mutant sequence TCL1G conjugated to a transactivator of transcription peptide sequence to promote intracellular uptake. Four groups of mice were studied, enteral nutrition group (control), control mice given a functioning TCL1 (control + TCL1), TPN mice given TCL1G (control peptide, TPN + TCL1G); and TPN mice given TCL1. TPN mice given TCL1G showed a significant decrease in jejunal EC p-Akt (Ser473 and Thr308) abundance, whereas TPN + TCL1 mice showed increased p-Akt (Ser473) abundance. Phosphorylation of beta-catenin and glycogen synthase kinase-3beta (downstream targets of Akt signaling) were also decreased in the TPN + TCL1G group and completely prevented in the TPN + TCL1 group. Use of TCL1 nearly completely prevented the decline in EC proliferation seen in the TPN + TCL1G group, as well as partly returned EC apoptosis levels close to controls. The mammalian target of rapamycin pathway demonstrated a similar reduction in activity in the TPN + TCL1G group that was significantly prevented in the TPN + TCL1 group. These results support a significant loss of PI3K/p-Akt signaling upon replacing enteral nutrition with TPN, and prevention of this loss demonstrates the key importance of PI3K/p-Akt signaling in maintaining gut integrity including EC proliferation and reduction in apoptosis.
Figures




References
-
- Auguin D, Barthe P, Royer C, Stern M, Noguchi M, Arold S, Roumestand C. Structural basis for the co-activation of protein kinase B by T-cell Leukemia-1 (TCL1) family proto-oncoproteins. J Cell Biol 27: 35890–35902, 2004 - PubMed
-
- Blachier F, Boutry C, Bos C, Tomé D. Metabolism and functions of L-glutamate in the epithelial cells of the small and large intestines. Am J Clin Nutr 90: 814S–821S, 2009 - PubMed
-
- Chiu T, Santiskulvong C, Rozengurt E. EGF receptor transactivation mediates ANG II-stimulated mitogenesis in intestinal epithelial cells through the PI3-kinase/Akt/mTOR/p70S6K1 signaling pathway. Am J Physiol Gastrointest Liver Physiol 288: G182–G194, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

