Regression of advanced rat and human gliomas by local or systemic treatment with oncolytic parvovirus H-1 in rat models
- PMID: 20299703
- PMCID: PMC2940670
- DOI: 10.1093/neuonc/noq023
Regression of advanced rat and human gliomas by local or systemic treatment with oncolytic parvovirus H-1 in rat models
Abstract
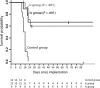
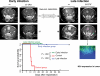
Oncolytic virotherapy is a potential treatment modality under investigation for various malignancies including malignant brain tumors. Unlike some other natural or modified viruses that show oncolytic activity against cerebral neoplasms, the rodent parvovirus H-1 (H-1PV) is completely apathogenic in humans. H-1PV efficiently kills a number of tumor cells without harm to corresponding normal ones. In this study, the concept of H-1PV-based virotherapy of glioma was tested for rat (RG-2 cell-derived) and for human (U87 cell-derived) gliomas in immunocompetent and immunodeficient rat models, respectively. Large orthotopic rat and human glioma cell-derived tumors were treated with either single stereotactic intratumoral or multiple intravenous (iv) H-1PV injections. Oncolysis was monitored by magnetic resonance imaging and proven by histology. Virus distribution and replication were determined in brain and organs. In immunocompetent rats bearing RG-2-derived tumors, a single stereotactic intratumoral injection of H-1PV and multiple systemic (iv) applications of the virus were sufficient for remission of advanced and even symptomatic intracranial gliomas without damaging normal brain tissue or other organs. H-1PV therapy resulted in significantly improved survival (Kaplan-Meier analysis) in both the rat and human glioma models. Virus replication in tumors indicated a contribution of secondary infection by progeny virus to the efficiency of oncolysis. Virus replication was restricted to tumors, although H-1PV DNA could be detected transiently in adjacent or remote normal brain tissue and in noncerebral tissues. The results presented here and the innocuousness of H-1PV for humans argue for the use of H-1PV as a powerful means to perform oncolytic therapy of malignant gliomas.
Figures



Similar articles
-
Regression of glioma in rat models by intranasal application of parvovirus h-1.Clin Cancer Res. 2011 Aug 15;17(16):5333-42. doi: 10.1158/1078-0432.CCR-10-3124. Epub 2011 Jun 29. Clin Cancer Res. 2011. PMID: 21715567
-
Therapeutic implications of the enhanced short and long-term cytotoxicity of radiation treatment followed by oncolytic parvovirus H-1 infection in high-grade glioma cells.Bioeng Bugs. 2010 Nov-Dec;1(6):429-33. doi: 10.4161/bbug.1.6.12943. Bioeng Bugs. 2010. PMID: 21468212 Free PMC article.
-
Improved killing of human high-grade glioma cells by combining ionizing radiation with oncolytic parvovirus H-1 infection.J Biomed Biotechnol. 2010;2010:350748. doi: 10.1155/2010/350748. Epub 2010 Mar 7. J Biomed Biotechnol. 2010. PMID: 20224643 Free PMC article.
-
Virotherapy of digestive tumors with rodent parvovirus: overview and perspectives.Expert Opin Biol Ther. 2016;16(5):645-53. doi: 10.1517/14712598.2016.1151492. Epub 2016 Mar 28. Expert Opin Biol Ther. 2016. PMID: 26855087 Review.
-
H-1 Parvovirus as a Cancer-Killing Agent: Past, Present, and Future.Viruses. 2019 Jun 18;11(6):562. doi: 10.3390/v11060562. Viruses. 2019. PMID: 31216641 Free PMC article. Review.
Cited by
-
Immune Suppression during Oncolytic Virotherapy for High-Grade Glioma; Yes or No?J Cancer. 2015 Jan 15;6(3):203-17. doi: 10.7150/jca.10640. eCollection 2015. J Cancer. 2015. PMID: 25663937 Free PMC article. Review.
-
Immune Conversion of Tumor Microenvironment by Oncolytic Viruses: The Protoparvovirus H-1PV Case Study.Front Immunol. 2019 Aug 7;10:1848. doi: 10.3389/fimmu.2019.01848. eCollection 2019. Front Immunol. 2019. PMID: 31440242 Free PMC article. Review.
-
Immunotherapeutic Potential of Oncolytic H-1 Parvovirus: Hints of Glioblastoma Microenvironment Conversion towards Immunogenicity.Viruses. 2017 Dec 15;9(12):382. doi: 10.3390/v9120382. Viruses. 2017. PMID: 29244745 Free PMC article.
-
The parvoviral capsid controls an intracellular phase of infection essential for efficient killing of stepwise-transformed human fibroblasts.Virology. 2011 Jul 20;416(1-2):32-41. doi: 10.1016/j.virol.2011.04.015. Epub 2011 May 20. Virology. 2011. PMID: 21600623 Free PMC article.
-
Intravenous injection of oncolytic picornavirus SVV-001 prolongs animal survival in a panel of primary tumor-based orthotopic xenograft mouse models of pediatric glioma.Neuro Oncol. 2013 Sep;15(9):1173-85. doi: 10.1093/neuonc/not065. Epub 2013 May 7. Neuro Oncol. 2013. PMID: 23658322 Free PMC article.
References
-
- Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. - PubMed
-
- Everts B, van der Poel HG. Replication-selective oncolytic viruses in the treatment of cancer. Cancer Gene Ther. 2005;12:141–161. - PubMed
-
- Lun X, Senger DL, Alain T, Oprea A, Parato K, Stojdl D, et al. Effects of intravenously administered recombinant vesicular stomatitis virus (VSV(deltaM51)) on multifocal and invasive gliomas. J Natl Cancer Inst. 2006;98:1546–1557. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

