The IKK complex, a central regulator of NF-kappaB activation
- PMID: 20300203
- PMCID: PMC2829958
- DOI: 10.1101/cshperspect.a000158
The IKK complex, a central regulator of NF-kappaB activation
Abstract
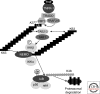
The IKK kinase complex is the core element of the NF-kappaB cascade. It is essentially made of two kinases (IKKalpha and IKKbeta) and a regulatory subunit, NEMO/IKKgamma. Additional components may exist, transiently or permanently, but their characterization is still unsure. In addition, it has been shown that two separate NF-kappaB pathways exist, depending on the activating signal and the cell type, the canonical (depending on IKKbeta and NEMO) and the noncanonical pathway (depending solely on IKKalpha). The main question, which is still only partially answered, is to understand how an NF-kappaB activating signal leads to the activation of the kinase subunits, allowing them to phosphorylate their targets and eventually induce nuclear translocation of the NF-kappaB dimers. I will review here the genetic, biochemical, and structural data accumulated during the last 10 yr regarding the function of the three IKK subunits.
Figures
References
-
- Abbott DW, Wilkins A, Asara JM, Cantley LC 2004. The Crohn’s disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr Biol 14:2217–2227 - PubMed
-
- Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS 2003. A nucleosomal function for IkB kinase a (IKKa) is essential for NF-kB dependent gene expression. Nature 423:659–663 - PubMed
-
- Bagnéris C, Ageichik AV, Cronin N, Wallace B, Collins M, Boshoff C, Waksman G, Barrett T 2008. Crystal structure of a vFlip-IKKγ complex: Insights into viral activation of the IKK signalosome. Molecular Cell 30:620–631 - PubMed
-
- Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV, Karin M 2001. IKKα Provides an Essential Link between RANK Signaling and Cyclin D1 Expression during Mammary Gland Development. Cell 107:763–775 - PubMed
-
- Carter RS, Pennington KN, Ungurait BJ, Ballard DW 2003. In Vivo Identification of Inducible Phosphoacceptors in the IKKγ/NEMO Subunit of Human IκB Kinase. J Biol Chem 278:19642–19648 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
