ICAM-1 clustering on endothelial cells recruits VCAM-1
- PMID: 20300427
- PMCID: PMC2840377
- DOI: 10.1155/2010/120328
ICAM-1 clustering on endothelial cells recruits VCAM-1
Abstract
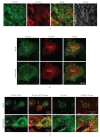
In the initial stages of transendothelial migration, leukocytes use the endothelial integrin ligands ICAM-1 and VCAM-1 for strong adhesion. Upon adhesion of the leukocyte to endothelial ICAM-1, ICAM-1 is clustered and recruited to the adhered leukocyte, promoting strong adhesion. In this study, we provide evidence for the colocalization of VCAM-1 at sites of ICAM-1 clustering. Anti-ICAM-1 antibody-coated beads were used to selectively cluster and recruit ICAM-1 on primary human endothelial cells. In time, co-localization of ICAM-1 and VCAM-1 around the adherent beads was observed. Biochemical pull-down assays showed that ICAM-1 clustering induced its association to VCAM-1, suggesting a physical link between these two adhesion molecules. The association was partly dependent on lipid rafts as well as on F-actin and promoted adhesion. These data show that VCAM-1 can be recruited, in an integrin-independent fashion, to clustered ICAM-1 which may serve to promote ICAM-1-mediated leukocyte adhesion.
Figures



References
-
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76(2):301–314. - PubMed
-
- Kluger MS. Vascular endothelial cell adhesion and signaling during leukocyte recruitment. Advances in Dermatology. 2004;20:163–201. - PubMed
-
- van Buul JD, Mul FPJ, van der Schoot CE, Hordijk PL. ICAM-3 activation modulates cell-cell contacts of human bone marrow endothelial cells. Journal of Vascular Research. 2004;41(1):28–37. - PubMed
-
- Tilghman RW, Hoover RL. E-selectin and ICAM-1 are incorporated into detergent-insoluble membrane domains following clustering in endothelial cells. FEBS Letters. 2002;525(1–3):83–87. - PubMed
-
- Viola A, Gupta N. Tether and trap: regulation of membrane-raft dynamics by actin-binding proteins. Nature Reviews Immunology. 2007;7(11):889–896. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

