In vivo accumulation of Helicobacter pylori products, NOD1, ubiquitinated proteins and proteasome in a novel cytoplasmic structure
- PMID: 20300534
- PMCID: PMC2838800
- DOI: 10.1371/journal.pone.0009716
In vivo accumulation of Helicobacter pylori products, NOD1, ubiquitinated proteins and proteasome in a novel cytoplasmic structure
Abstract
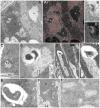
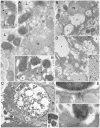
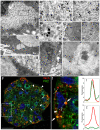
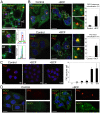
Cell internalization and intracellular fate of H. pylori products/virulence factors in vivo by human gastric epithelium, the main target of H. pylori-induced pathologies (i.e., peptic ulcer and cancer), are still largely unknown. Investigating gastric endoscopic biopsies from dyspeptic patients by means of ultrastructural immunocytochemistry, here we show that, in human superficial-foveolar epithelium and its metaplastic or dysplastic foci, H. pylori virulence factors accumulated in a discrete cytoplasmic structure characterized by 13-nm-thick cylindrical particles of regular punctate-linear substructure resembling the proteasome complex in size and structure. Inside this particle-rich cytoplasmic structure (PaCS) we observed colocalization of VacA, CagA, urease and outer membrane proteins with NOD1 receptor, ubiquitin-activating enzyme E1, polyubiquitinated proteins, proteasome components and potentially oncogenic proteins like SHP2 and ERKs in human gastric epithelium. By means of electron and confocal microscopy, we demonstrate that the in vivo findings were reproduced in vitro by incubating human epithelial cell lines with H. pylori products/virulence factors. PaCSs differed from VacA-induced vacuoles, phagosomes, aggresomes or related bodies. Our data suggest that PaCS is a novel, proteasome-enriched structure arising in ribosome-rich cytoplasm at sites of H. pylori products accumulation. As a site of selective concentration of bacterial virulence factors, the ubiquitin-proteasome system and interactive proteins, PaCS is likely to modulate immune-inflammatory and proliferative responses of the gastric epithelium of potential pathologic relevance.
Conflict of interest statement
Figures



Similar articles
-
Proteasome particle-rich structures are widely present in human epithelial neoplasms: correlative light, confocal and electron microscopy study.PLoS One. 2011;6(6):e21317. doi: 10.1371/journal.pone.0021317. Epub 2011 Jun 17. PLoS One. 2011. PMID: 21695063 Free PMC article.
-
CagA Effector Protein in Helicobacter pylori-Infected Human Gastric Epithelium in Vivo: From Bacterial Core and Adhesion/Injection Clusters to Host Cell Proteasome-Rich Cytosol.Toxins (Basel). 2019 Oct 25;11(11):618. doi: 10.3390/toxins11110618. Toxins (Basel). 2019. PMID: 31731531 Free PMC article.
-
KCTD5 and Ubiquitin Proteasome Signaling Are Required for Helicobacter pylori Adherence.Front Cell Infect Microbiol. 2017 Oct 24;7:450. doi: 10.3389/fcimb.2017.00450. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29114497 Free PMC article.
-
Proteasome-Rich PaCS as an Oncofetal UPS Structure Handling Cytosolic Polyubiquitinated Proteins. In Vivo Occurrence, in Vitro Induction, and Biological Role.Int J Mol Sci. 2018 Sep 14;19(9):2767. doi: 10.3390/ijms19092767. Int J Mol Sci. 2018. PMID: 30223470 Free PMC article. Review.
-
Nucleotide-binding oligomerization domain 1 and Helicobacter pylori infection: A review.World J Gastroenterol. 2018 Apr 28;24(16):1725-1733. doi: 10.3748/wjg.v24.i16.1725. World J Gastroenterol. 2018. PMID: 29713127 Free PMC article. Review.
Cited by
-
Particulate cytoplasmic structures with high concentration of ubiquitin-proteasome accumulate in myeloid neoplasms.J Hematol Oncol. 2015 Jun 18;8:71. doi: 10.1186/s13045-015-0169-6. J Hematol Oncol. 2015. PMID: 26081257 Free PMC article.
-
Association of NOD1 and NOD2 genes polymorphisms with Helicobacter pylori related gastric cancer in a Chinese population.World J Gastroenterol. 2012 May 7;18(17):2112-20. doi: 10.3748/wjg.v18.i17.2112. World J Gastroenterol. 2012. PMID: 22563200 Free PMC article.
-
Lysosomes Dysfunction Causes Mitophagy Impairment in PBMCs of Sporadic ALS Patients.Cells. 2022 Apr 9;11(8):1272. doi: 10.3390/cells11081272. Cells. 2022. PMID: 35455952 Free PMC article.
-
VacA generates a protective intracellular reservoir for Helicobacter pylori that is eliminated by activation of the lysosomal calcium channel TRPML1.Nat Microbiol. 2019 Aug;4(8):1411-1423. doi: 10.1038/s41564-019-0441-6. Epub 2019 May 20. Nat Microbiol. 2019. PMID: 31110360 Free PMC article.
-
Chaperone molecules concentrate together with the ubiquitin-proteasome system inside particulate cytoplasmic structures: possible role in metabolism of misfolded proteins.Histochem Cell Biol. 2015 Aug;144(2):179-84. doi: 10.1007/s00418-015-1327-1. Epub 2015 May 8. Histochem Cell Biol. 2015. PMID: 25952156
References
-
- Chan WY, Hui PK, Leung KM, Thomas TM. Modes of Helicobacter colonization and gastric epithelial damage. Histopathology. 1992;21:521–528. - PubMed
-
- Necchi V, Candusso ME, Tava F, Luinetti O, Ventura U, et al. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology. 2007;132:1009–1023. - PubMed
-
- Amieva MR, Salama NR, Tompkins LS, Falkow S. Helicobacter pylori enter and survive within multivesicular vacuoles of epithelial cells. Cell Microbiol. 2002;4:677–690. - PubMed
-
- Odenbreit S, Püls J, Sedlmaier B, Gerland E, Fischer W, et al. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

