FOXO1 plays an essential role in apoptosis of retinal pericytes
- PMID: 20300563
- PMCID: PMC2838737
FOXO1 plays an essential role in apoptosis of retinal pericytes
Abstract
Purpose: An early and significant event in diabetic retinopathy is the loss of retinal microvascular pericytes. Studies were performed to investigate pathways through which an advanced glycation endproduct and tumor necrosis factor (TNF)-alpha stimulate apoptosis in retinal pericytes through the activation of the pro-apoptotic transcription factor Forkhead box O1 (FOXO1).
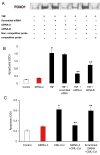
Methods: Human retinal pericytes were stimulated by carboxymethyllysine (CML)-collagen, an advanced glycation endproduct, or TNF-alpha in vitro. Apoptosis was assessed by measuring cytoplasmic histone-associated DNA. The role of FOXO1 was examined by RNA interference (RNAi), and specific inhibitors were used to investigate the role of p38 and Jun N-terminal kinase mitogen-activated protein kinase (JNK MAP) kinases, Akt, and nuclear factor kappa B (NF-kappaB). Caspase-3 activity was measured with a luminescent substrate, and FOXO1 DNA-binding activity was measured by electrophoretic mobility shift assay (EMSA).
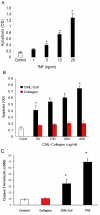
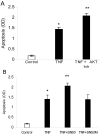
Results: TNF-alpha and CML-collagen but not control collagen stimulated apoptosis, caspase-3 activity, and FOXO1 DNA-binding activity in pericytes. Silencing FOXO1 by small interfering RNA prevented apoptosis of pericytes in response to both TNF-alpha and CML-collagen. By use of specific inhibitors, we demonstrated that both FOXO1 activation and subsequent apoptosis was mediated, in part, by p38 and JNK MAP kinases. In contrast Akt and NF-kappaB inhibitors had the opposite effect on pericyte apoptosis.
Conclusions: The results demonstrate pathways through which two different mediators, TNF-alpha and an advanced glycation endproduct, can induce pericyte apoptosis through activation of the transcription factor FOXO1.
Figures
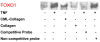

References
-
- Singleton JR, Smith AG, Russell JW, Feldman EL. Microvascular complications of impaired glucose tolerance. Diabetes. 2003;52:2867–73. - PubMed
-
- Oshitari T, Polewski P, Chadda M, Li AF, Sato T, Roy S. Effect of combined antisense oligonucleotides against high-glucose- and diabetes-induced overexpression of extracellular matrix components and increased vascular permeability. Diabetes. 2006;55:86–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
