Membrane damage elicits an immunomodulatory program in Staphylococcus aureus
- PMID: 20300601
- PMCID: PMC2837406
- DOI: 10.1371/journal.ppat.1000802
Membrane damage elicits an immunomodulatory program in Staphylococcus aureus
Abstract
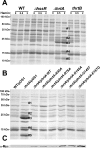
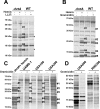
The Staphylococcus aureus HrtAB system is a hemin-regulated ABC transporter composed of an ATPase (HrtA) and a permease (HrtB) that protect S. aureus against hemin toxicity. S. aureus strains lacking hrtA exhibit liver-specific hyper-virulence and upon hemin exposure over-express and secrete immunomodulatory factors that interfere with neutrophil recruitment to the site of infection. It has been proposed that heme accumulation in strains lacking hrtAB is the signal which triggers S. aureus to elaborate this anti-neutrophil response. However, we report here that S. aureus strains expressing catalytically inactive HrtA do not elaborate the same secreted protein profile. This result indicates that the physical absence of HrtA is responsible for the increased expression of immunomodulatory factors, whereas deficiencies in the ATPase activity of HrtA do not contribute to this process. Furthermore, HrtB expression in strains lacking hrtA decreases membrane integrity consistent with dysregulated permease function. Based on these findings, we propose a model whereby hemin-mediated over-expression of HrtB in the absence of HrtA damages the staphylococcal membrane through pore formation. In turn, S. aureus senses this membrane damage, triggering the increased expression of immunomodulatory factors. In support of this model, wildtype S. aureus treated with anti-staphylococcal channel-forming peptides produce a secreted protein profile that mimics the effect of treating DeltahrtA with hemin. These results suggest that S. aureus senses membrane damage and elaborates a gene expression program that protects the organism from the innate immune response of the host.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
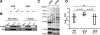



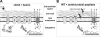
References
-
- Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet. 2004;364:703–705. - PubMed
-
- Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin Infect Dis. 2001;32(Suppl 2):S114–132. - PubMed
-
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. - PubMed
-
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

