Influence of maternal gestational treatment with mycobacterial antigens on postnatal immunity in an experimental murine model
- PMID: 20300629
- PMCID: PMC2837747
- DOI: 10.1371/journal.pone.0009699
Influence of maternal gestational treatment with mycobacterial antigens on postnatal immunity in an experimental murine model
Abstract
Background: It has been proposed that the immune system could be primed as early as during the fetal life and this might have an impact on postnatal vaccination. Therefore, we addressed in murine models whether gestational treatment with mycobacterial antigens could induce better immune responses in the postnatal life.
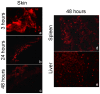
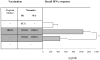
Methods/findings: BALB/c mice were treated subcutaneously (s.c.) at the second week of gestation with antigen (Ag)85A or heparin-binding hemagglutinin (HBHA) in the absence of adjuvant. Following birth, offspring mice were immunized intranasally (i.n.) with the same antigens formulated with the adjuvant cholera toxin (CT) at week 1 and week 4. One week after the last immunization, we assessed antigen-specific recall interferon gamma (IFN-gamma) responses by in vitro restimulation of lung-derived lymphocytes. Protection against infection was assessed by challenge with high dose Mycobacterium bovis Bacille Calmette-Guérin (BCG) given i.n. We found that recall IFN-gamma responses were higher in the offspring born to the treated mother compared to the untreated-mother. More importantly, we observed that the offspring born to the treated mother controlled infection better than the offspring born to the untreated mother. Since the gestational treatment was done in absence of adjuvant, essentially there was no antibody production observed in the pregnant mice and therefore no influence of maternal antibodies was expected. We hypothesized that the effect of maternal treatment with antigen on the offspring occurred due to antigen transportation through placenta. To trace the antigens, we conjugated fluorescent nanocrystals with Ag85A (Qdot-ITK-Ag85A). After inoculation in the pregnant mice, Qdot-ITK-Ag85A conjugates were detected in the liver, spleen of pregnant females and in all the fetuses and placentas examined.
Conclusion: The fetal immune system could be primed in utero by mycobacterial antigens transported through the placenta.
Conflict of interest statement
Figures

Similar articles
-
Mucosal immunization with recombinant heparin-binding haemagglutinin adhesin suppresses extrapulmonary dissemination of Mycobacterium bovis bacillus Calmette-Guérin (BCG) in infected mice.Vaccine. 2008 Feb 13;26(7):924-32. doi: 10.1016/j.vaccine.2007.12.005. Epub 2007 Dec 26. Vaccine. 2008. PMID: 18192091
-
A DNA prime-live vaccine boost strategy in mice can augment IFN-gamma responses to mycobacterial antigens but does not increase the protective efficacy of two attenuated strains of Mycobacterium bovis against bovine tuberculosis.Immunology. 2003 Apr;108(4):548-55. doi: 10.1046/j.1365-2567.2003.01589.x. Immunology. 2003. PMID: 12667217 Free PMC article.
-
Enhanced effect of BCG vaccine against pulmonary Mycobacterium tuberculosis infection in mice with lung Th17 response to mycobacterial heparin-binding hemagglutinin adhesin antigen.Microbiol Immunol. 2015 Dec;59(12):735-43. doi: 10.1111/1348-0421.12340. Microbiol Immunol. 2015. PMID: 26577130
-
Priming but not boosting with plasmid DNA encoding mycolyl-transferase Ag85A from Mycobacterium tuberculosis increases the survival time of Mycobacterium bovis BCG vaccinated mice against low dose intravenous challenge with M. tuberculosis H37Rv.Vaccine. 2006 Apr 12;24(16):3353-64. doi: 10.1016/j.vaccine.2005.12.066. Epub 2006 Feb 6. Vaccine. 2006. PMID: 16488518
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
Cited by
-
Fetal Macrophages Exposed to Salmonella Antigens Elicit Protective Immunity Against Overwhelming Salmonella Challenge in A Murine Model.Biomedicines. 2021 Mar 1;9(3):245. doi: 10.3390/biomedicines9030245. Biomedicines. 2021. PMID: 33804435 Free PMC article.
-
The impact of HIV exposure and maternal Mycobacterium tuberculosis infection on infant immune responses to bacille Calmette-Guérin vaccination.AIDS. 2015 Jan 14;29(2):155-65. doi: 10.1097/QAD.0000000000000536. AIDS. 2015. PMID: 25535752 Free PMC article.
-
Effects of maternal and infant co-infections, and of maternal immunisation, on the infant response to BCG and tetanus immunisation.Vaccine. 2010 Dec 16;29(2):247-55. doi: 10.1016/j.vaccine.2010.10.047. Epub 2010 Oct 30. Vaccine. 2010. PMID: 21040693 Free PMC article.
-
Maternal immunity enhances Mycoplasma hyopneumoniae vaccination induced cell-mediated immune responses in piglets.BMC Vet Res. 2014 Jun 5;10:124. doi: 10.1186/1746-6148-10-124. BMC Vet Res. 2014. PMID: 24903770 Free PMC article.
-
Beyond Passive Immunity: Is There Priming of the Fetal Immune System Following Vaccination in Pregnancy and What Are the Potential Clinical Implications?Front Immunol. 2018 Jul 16;9:1548. doi: 10.3389/fimmu.2018.01548. eCollection 2018. Front Immunol. 2018. PMID: 30061881 Free PMC article. Review.
References
-
- Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–80. - PubMed
-
- Hesseling AC, Rabie H, Marais BJ, Manders M, Lips M, et al. Bacille Calmette-Guérin vaccine-induced disease in HIV-infected and HIV-uninfected children. Clin Infect Dis. 2006;42:548–58. - PubMed
-
- Sarzotti M, Robbins DS, Hoffman PM. Induction of protective CTL responses in newborn mice by a murine retrovirus. Science. 1996;271:1726–8. - PubMed
-
- Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–46. - PubMed
-
- Adkins B. T-cell function in newborn mice and humans. Immunol Today. 1999;20:330–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

