Arginylation-dependent neural crest cell migration is essential for mouse development
- PMID: 20300656
- PMCID: PMC2837401
- DOI: 10.1371/journal.pgen.1000878
Arginylation-dependent neural crest cell migration is essential for mouse development
Abstract
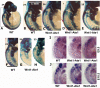
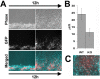
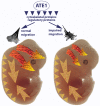
Coordinated cell migration during development is crucial for morphogenesis and largely relies on cells of the neural crest lineage that migrate over long distances to give rise to organs and tissues throughout the body. Recent studies of protein arginylation implicated this poorly understood posttranslational modification in the functioning of actin cytoskeleton and in cell migration in culture. Knockout of arginyltransferase (Ate1) in mice leads to embryonic lethality and severe heart defects that are reminiscent of cell migration-dependent phenotypes seen in other mouse models. To test the hypothesis that arginylation regulates cell migration during morphogenesis, we produced Wnt1-Cre Ate1 conditional knockout mice (Wnt1-Ate1), with Ate1 deletion in the neural crest cells driven by Wnt1 promoter. Wnt1-Ate1 mice die at birth and in the first 2-3 weeks after birth with severe breathing problems and with growth and behavioral retardation. Wnt1-Ate1 pups have prominent defects, including short palate and altered opening to the nasopharynx, and cranial defects that likely contribute to the abnormal breathing and early death. Analysis of neural crest cell movement patterns in situ and cell motility in culture shows an overall delay in the migration of Ate1 knockout cells that is likely regulated by intracellular mechanisms rather than extracellular signaling events. Taken together, our data suggest that arginylation plays a general role in the migration of the neural crest cells in development by regulating the molecular machinery that underlies cell migration through tissues and organs during morphogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Bronner-Fraser M, Fraser SE. Cell lineage analysis of the avian neural crest. Development. 1991;Suppl 2:17–22. - PubMed
-
- Fraser SE. Pattern formation in the vertebrate nervous system. Curr Opin Genet Dev. 1991;1:217–220. - PubMed
-
- Le Douarin NM. The Neural Crest (Developmental and Cell Biology Series): Cambridge University Press. 1983.
-
- Tucker RP. Neural crest cells: a model for invasive behavior. Int J Biochem Cell Biol. 2004;36:173–177. - PubMed
-
- Saint-Jeannet J-P, editor. New Yory, NY: Springer Science + Business Media, LLC; 2006. Neural Crest Induction and Differentiation.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

