Sentinel node identification rate and nodal involvement in the EORTC 10981-22023 AMAROS trial
- PMID: 20300966
- PMCID: PMC2889289
- DOI: 10.1245/s10434-010-0945-z
Sentinel node identification rate and nodal involvement in the EORTC 10981-22023 AMAROS trial
Abstract
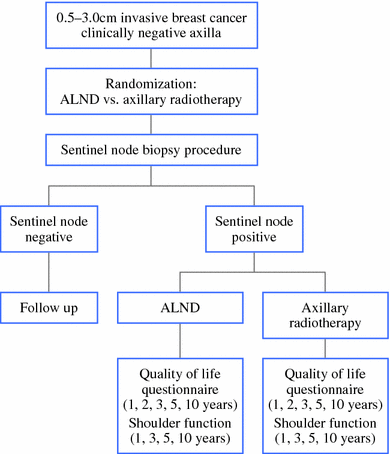
Background: The randomized EORTC 10981-22023 AMAROS trial investigates whether breast cancer patients with a tumor-positive sentinel node biopsy (SNB) are best treated with an axillary lymph node dissection (ALND) or axillary radiotherapy (ART). The aim of the current substudy was to evaluate the identification rate and the nodal involvement.
Methods: The first 2,000 patients participating in the AMAROS trial were evaluated. Associations between the identification rate and technical, patient-, and tumor-related factors were evaluated. The outcome of the SNB procedure and potential further nodal involvement was assessed.
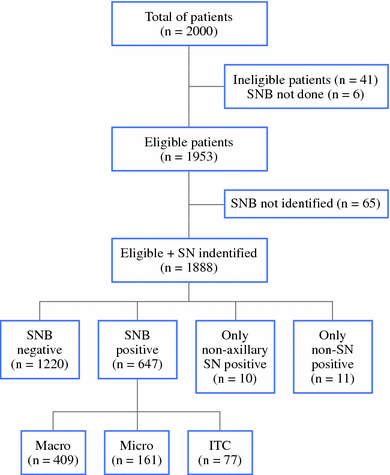
Results: In 65 patients, the sentinel node could not be identified. As a result, the sentinel node identification rate was 97% (1,888 of 1,953). Variables affecting the success rate were age, pathological tumor size, histology, year of accrual, and method of detection. The SNB results of 65% of the patients (n = 1,220) were negative and the patients underwent no further axillary treatment. The SNB results were positive in 34% of the patients (n = 647), including macrometastases (n = 409, 63%), micrometastases (n = 161, 25%), and isolated tumor cells (n = 77, 12%). Further nodal involvement in patients with macrometastases, micrometastases, and isolated tumor cells undergoing an ALND was 41, 18, and 18%, respectively.
Conclusions: With a 97% detection rate in this prospective international multicenter study, the SNB procedure is highly effective, especially when the combined method is used. Further nodal involvement in patients with micrometastases and isolated tumor cells in the sentinel node was similar-both were 18%.
Figures

References
-
- Braithwaite LR. The flow of lymph from the ileocaecal angle, and its possible bearing on the cause of duodenal and gastric ulcer. Br J Surg. 1923;11:7–26. doi: 10.1002/bjs.1800114103. - DOI
-
- van der Ploeg IM, Nieweg OE, van Rijk MC, Valdes Olmos RA, Kroon BB. Axillary recurrence after a tumour-negative sentinel node biopsy in breast cancer patients: a systematic review and meta-analysis of the literature. Eur J Surg Oncol. 2008;34:1277–1284. - PubMed
-
- Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–888. doi: 10.1016/S1470-2045(07)70278-4. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

