Ladder polyether synthesis via epoxide-opening cascades directed by a disappearing trimethylsilyl group
- PMID: 20302314
- PMCID: PMC3018858
- DOI: 10.1021/jo1002455
Ladder polyether synthesis via epoxide-opening cascades directed by a disappearing trimethylsilyl group
Abstract
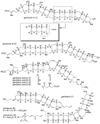
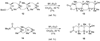
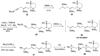
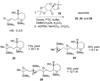
Epoxide-opening cascades offer the potential to construct complex polyether natural products expeditiously and in a manner that emulates the biogenesis proposed for these compounds. Herein we provide a full account of our development of a strategy that addresses several important challenges of such cascades. The centerpiece of the method is a trimethylsilyl (SiMe(3)) group that serves several purposes and leaves no trace of itself by the time the cascade has come to an end. The main function of the SiMe(3) group is to dictate the regioselectivity of epoxide opening. This strategy is the only general method of effecting endo-selective cascades under basic conditions.
Figures


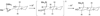

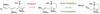

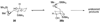
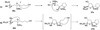




















References
-
-
For reviews on ladder ether synthesis: Sasaki M, Fuwa H. Nat. Prod. Rep. 2008;25:401–426. Nakata T. Chem Rev. 2005;105:4314–4347. Alvarez E, Candenas M-L, Pérez R, Ravelo JL, Martín JD. Chem. Rev. 1995;95:1953–1980. Hoberg JO. Tetrahedron. 1998;54:12631–12670. Marmsäter FP, West FG. Chem. Eur. J. 2002;8:4347–4353. Hirama M, Rainier JD, editors. Tetrahedron Symposium-in-Print 90. Vol. 58. 2002. pp. 1779–2040. Evans PA, Delouvrie B. Curr. Opin. Drug Discovery Dev. 2002;5:986–999. Vilotijevic I, Jamison TF, editors. Angew. Chem. Int. Ed. Vol. 48. 2009. pp. 5250–5281. Morten CJ, Byers JA, Van Dyke AR, Vilotijevic I, Jamison TF. Chem. Soc. Rev. 2009;38:3175–3192.
-
-
- Gallimore AR, Spencer JB. Angew. Chem., Int. Ed. 2006;45:4406–4413. - PubMed
- Nicolaou KC, Frederick MO. Angew. Chem., Int. Ed. 2007;46:5278–5282. - PubMed
- Nicolaou KC, Frederick MO, Burtoloso ACB, Denton RM, Rivas F, Cole KP, Aversa RJ, Gibe R, Umezawa T, Suzuki T. J. Am. Chem. Soc. 2008;130:7466–7476. - PubMed
-
-
Nakanishi K. Toxicon. 1985;23:473–479. Lee MS, Repeta DJ, Nakanishi K, Zagorski MG. J. Am. Chem. Soc. 1986;108:7855–7856. See also: Chou H-N, Shimizu Y. J. Am. Chem. Soc. 1987;109:2184–2185.
-
-
- Coxon JM, Hartshorn MP, Swallow WH. Aust. J. Chem. 1973;26:2521–2526.
-
- Nicolaou KC, Duggan ME, Hwang C-K, Somers PK. J. Chem. Soc., Chem. Commun. 1985:1359–1362.
- Nicolaou KC, Prasad CVC, Somers PK, Hwang C-K. J. Am. Chem. Soc. 1989;111:5330–5334.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

