Differential effects of Paclitaxel on dendritic cell function
- PMID: 20302610
- PMCID: PMC2850888
- DOI: 10.1186/1471-2172-11-14
Differential effects of Paclitaxel on dendritic cell function
Abstract
Background: The potential utility of dendritic cells (DC) as cancer vaccines has been established in early trials in human cancers. The concomitant administration of cytotoxic agents and DC vaccines has been previously avoided due to potential immune suppression by chemotherapeutics. Recent studies show that common chemotherapy agents positively influence adaptive and innate anti-tumour immune responses.
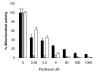
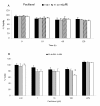
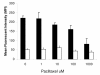
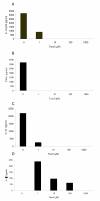
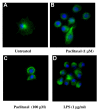
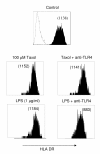
Results: We investigated the effects of paclitaxel on human DC biology in vitro. DCs appear to sustain a significant level of resistance to paclitaxel and maintain normal viability at concentrations of up to 100 micromol. In some cases this resistance against paclitaxel is significantly better than the level seen in tumour cell lines. Paclitaxel exposure led to a dose dependent increase in HLA class II expression equivalent to exposure to lipopolysaccharide (LPS), and a corresponding increase in proliferation of allogeneic T cells at the clinically relevant doses of paclitaxel. Increase in HLA-Class II expression induced by paclitaxel was not blocked by anti TLR-4 antibody. However, paclitaxel exposure reduced the endocytic capacity of DC but reduced the expression of key pro-inflammatory cytokines such as IL-12 and TNFalpha. Key morphological changes occurred when immature DC were cultured with 100 micromol paclitaxel. They became small rounded cells with stable microtubules, whereas there were little effects on LPS-matured DC.
Conclusions: The effect of paclitaxel on human monocyte derived DC is complex, but in the clinical context of patients receiving preloaded and matured DC vaccines, its immunostimulatory potential and resistance to direct cytotoxicity by paclitaxel would indicate potential advantages to co-administration with vaccines.
Figures
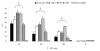
Similar articles
-
IL-21 enhances SOCS gene expression and inhibits LPS-induced cytokine production in human monocyte-derived dendritic cells.J Leukoc Biol. 2006 Jun;79(6):1279-85. doi: 10.1189/jlb.0905503. Epub 2006 Mar 21. J Leukoc Biol. 2006. PMID: 16551679
-
Piperine impairs the migration and T cell-activating function of dendritic cells.Toxicol Lett. 2016 Feb 3;242:23-33. doi: 10.1016/j.toxlet.2015.11.025. Epub 2015 Nov 27. Toxicol Lett. 2016. PMID: 26640239
-
The presence of interleukin-27 during monocyte-derived dendritic cell differentiation promotes improved antigen processing and stimulation of T cells.Immunology. 2015 Apr;144(4):649-60. doi: 10.1111/imm.12417. Immunology. 2015. PMID: 25346485 Free PMC article.
-
Fusion of Bacterial Flagellin to a Dendritic Cell-Targeting αCD40 Antibody Construct Coupled With Viral or Leukemia-Specific Antigens Enhances Dendritic Cell Maturation and Activates Peptide-Responsive T Cells.Front Immunol. 2020 Nov 12;11:602802. doi: 10.3389/fimmu.2020.602802. eCollection 2020. Front Immunol. 2020. PMID: 33281829 Free PMC article.
-
Aspirin inhibits in vitro maturation and in vivo immunostimulatory function of murine myeloid dendritic cells.J Immunol. 2001 Jun 15;166(12):7053-62. doi: 10.4049/jimmunol.166.12.7053. J Immunol. 2001. PMID: 11390449
Cited by
-
Nab-paclitaxel promotes the cancer-immunity cycle as a potential immunomodulator.Am J Cancer Res. 2021 Jul 15;11(7):3445-3460. eCollection 2021. Am J Cancer Res. 2021. PMID: 34354854 Free PMC article. Review.
-
Dendritic cells matured with recombinant human sperm associated antigen 9 (rhSPAG9) induce CD4+, CD8+ T cells and activate NK cells: a potential candidate molecule for immunotherapy in cervical cancer.Cancer Cell Int. 2021 Sep 7;21(1):473. doi: 10.1186/s12935-021-01951-7. Cancer Cell Int. 2021. PMID: 34493268 Free PMC article.
-
IL4 receptor targeting enables nab-paclitaxel to enhance reprogramming of M2-type macrophages into M1-like phenotype via ROS-HMGB1-TLR4 axis and inhibition of tumor growth and metastasis.Theranostics. 2024 Apr 8;14(6):2605-2621. doi: 10.7150/thno.92672. eCollection 2024. Theranostics. 2024. PMID: 38646639 Free PMC article.
-
Gastric cancer vaccines synthesized using a TLR7 agonist and their synergistic antitumor effects with 5-fluorouracil.J Transl Med. 2018 May 8;16(1):120. doi: 10.1186/s12967-018-1501-z. J Transl Med. 2018. PMID: 29739434 Free PMC article.
-
Synergistic antitumor immune response mediated by paclitaxel-conjugated nanohybrid oncolytic adenovirus with dendritic cell therapy.Front Immunol. 2024 May 21;15:1355566. doi: 10.3389/fimmu.2024.1355566. eCollection 2024. Front Immunol. 2024. PMID: 38835775 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

