Blood still kills: six strategies to further reduce allogeneic blood transfusion-related mortality
- PMID: 20303034
- PMCID: PMC7126657
- DOI: 10.1016/j.tmrv.2009.11.001
Blood still kills: six strategies to further reduce allogeneic blood transfusion-related mortality
Erratum in
- Transfus Med Rev. 2010 Jul;24(3):257
Abstract
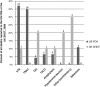
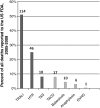
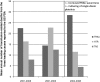
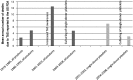
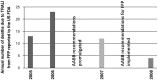
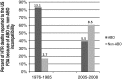
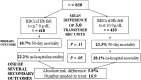
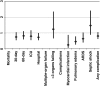
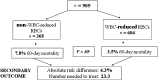
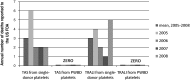
After reviewing the relative frequency of the causes of allogeneic blood transfusion-related mortality in the United States today, we present 6 possible strategies for further reducing such transfusion-related mortality. These are (1) avoidance of unnecessary transfusions through the use of evidence-based transfusion guidelines, to reduce potentially fatal (infectious as well as noninfectious) transfusion complications; (2) reduction in the risk of transfusion-related acute lung injury in recipients of platelet transfusions through the use of single-donor platelets collected from male donors, or female donors without a history of pregnancy or who have been shown not to have white blood cell (WBC) antibodies; (3) prevention of hemolytic transfusion reactions through the augmentation of patient identification procedures by the addition of information technologies, as well as through the prevention of additional red blood cell alloantibody formation in patients who are likely to need multiple transfusions in the future; (4) avoidance of pooled blood products (such as pooled whole blood-derived platelets) to reduce the risk of transmission of emerging transfusion-transmitted infections (TTIs) and the residual risk from known TTIs (especially transfusion-associated sepsis [TAS]); (5) WBC reduction of cellular blood components administered in cardiac surgery to prevent the poorly understood increased mortality seen in cardiac surgery patients in association with the receipt of non-WBC-reduced (compared with WBC-reduced) transfusion; and (6) pathogen reduction of platelet and plasma components to prevent the transfusion transmission of most emerging, potentially fatal TTIs and the residual risk of known TTIs (especially TAS).
(c) 2010 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention.Blood. 2009 Apr 9;113(15):3406-17. doi: 10.1182/blood-2008-10-167643. Epub 2009 Feb 2. Blood. 2009. PMID: 19188662 Review.
-
Risk-reduction strategies for platelet transfusion in the United States.ScientificWorldJournal. 2011 Mar 7;11:624-40. doi: 10.1100/tsw.2011.60. ScientificWorldJournal. 2011. PMID: 21403979 Free PMC article. Review.
-
[Single-donor (apheresis) platelets and pooled whole-blood-derived platelets--significance and assessment of both blood products].Clin Lab. 2014;60(4):S1-39. doi: 10.7754/clin.lab.2014.140210. Clin Lab. 2014. PMID: 24779310 Review. German.
-
The relative safety of pooled whole-blood-derived platelets prepared by the buffy-coat method versus single-donor (apheresis) platelets.Clin Lab. 2010;56(7-8):263-79. Clin Lab. 2010. PMID: 20857891 Review.
-
Rare antibody-associated hemolytic transfusion reaction and transfusion-related acute lung injury: a case report.BMC Surg. 2017 Apr 26;17(1):48. doi: 10.1186/s12893-017-0241-y. BMC Surg. 2017. PMID: 28441942 Free PMC article.
Cited by
-
Post-operative blood salvage in patient blood management: is it really cost-effective and safe?Blood Transfus. 2013 Apr;11(2):175-7. doi: 10.2450/2013.0001-13. Epub 2013 Feb 8. Blood Transfus. 2013. PMID: 23522880 Free PMC article. No abstract available.
-
Disclosure of physician-specific behavior improves blood utilization protocol adherence in cardiac surgery.Ann Thorac Surg. 2013 Dec;96(6):2168-74. doi: 10.1016/j.athoracsur.2013.06.080. Epub 2013 Sep 12. Ann Thorac Surg. 2013. PMID: 24035308 Free PMC article.
-
Blood management by transfusion triggers: when less is more.Blood Transfus. 2012 Jan;10(1):28-33. doi: 10.2450/2011.0108-10. Epub 2011 Jun 15. Blood Transfus. 2012. PMID: 21839021 Free PMC article.
-
Age of blood and recipient factors determine the severity of transfusion-related acute lung injury (TRALI).Crit Care. 2012 Feb 1;16(1):R19. doi: 10.1186/cc11178. Crit Care. 2012. PMID: 22297161 Free PMC article.
-
An Analysis of the Association of Whole Blood Transfusion With the Development of Acute Respiratory Distress Syndrome.Crit Care Med. 2025 Jan 1;53(1):e109-e116. doi: 10.1097/CCM.0000000000006477. Epub 2024 Nov 15. Crit Care Med. 2025. PMID: 39774204 Free PMC article.
References
-
- FDA/CBER: Fatalities reported to the FDA following blood collection and transfusion Annual summary for fiscal year 2008. http://www.fda.gov/cber/blood/SafetyAvailability/ReportaProblem/Transfus... Available at. Accessed September 21, 2009.
-
- Popovsky M.A., Moore S.B. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion. 1985;25:573–577. - PubMed
-
- Goldman M., Blajchman M.A. Blood products-associated bacterial sepsis. Transfus Med Rev. 1991;5:73–83. - PubMed
-
- Sazama K. Reports of 355 transfusion-associated deaths: 1976 through 1985. Transfusion. 1990;30:583–590. - PubMed
-
- Popovsky M.A., Chaplin H.C., Jr., Moore S.B. Transfusion-related acute lung injury: A neglected, serious complication of hemotherapy. Transfusion. 1992;32:589–592. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

