Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering
- PMID: 20303169
- PMCID: PMC2907908
- DOI: 10.1016/j.biomaterials.2010.02.044
Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering
Abstract
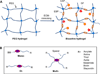
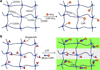
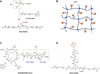
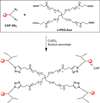
In this review, we explore different approaches for introducing bioactivity into poly(ethylene glycol) (PEG) hydrogels. Hydrogels are excellent scaffolding materials for repairing and regenerating a variety of tissues because they can provide a highly swollen three-dimensional (3D) environment similar to soft tissues. Synthetic hydrogels like PEG-based hydrogels have advantages over natural hydrogels, such as the ability for photopolymerization, adjustable mechanical properties, and easy control of scaffold architecture and chemical compositions. However, PEG hydrogels alone cannot provide an ideal environment to support cell adhesion and tissue formation due to their bio-inert nature. The natural extracellular matrix (ECM) has been an attractive model for the design and fabrication of bioactive scaffolds for tissue engineering. ECM-mimetic modification of PEG hydrogels has emerged as an important strategy to modulate specific cellular responses. To tether ECM-derived bioactive molecules (BMs) to PEG hydrogels, various strategies have been developed for the incorporation of key ECM biofunctions, such as specific cell adhesion, proteolytic degradation, and signal molecule-binding. A number of cell types have been immobilized on bioactive PEG hydrogels to provide fundamental knowledge of cell/scaffold interactions. This review addresses the recent progress in material designs and fabrication approaches leading to the development of bioactive hydrogels as tissue engineering scaffolds.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures









References
-
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. - PubMed
-
- Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliver Rev. 2002;43:3–12. - PubMed
-
- Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–1879. - PubMed
-
- Temenoff JS, Mikos AG. Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials. 2002;21:2405–2412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

