Diabetes mellitus impairs tendon-bone healing after rotator cuff repair
- PMID: 20303293
- PMCID: PMC5257255
- DOI: 10.1016/j.jse.2009.11.045
Diabetes mellitus impairs tendon-bone healing after rotator cuff repair
Abstract
Introduction: Studies have demonstrated a significant decrease in skeletal mass, bone mineral density, and impaired fracture healing in the diabetic population. However, the effect of sustained hyperglycemia on tendon-to-bone healing is unknown.
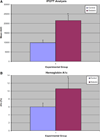
Materials and methods: Forty-eight male, Lewis rats underwent unilateral detachment of the supraspinatus tendon followed by immediate anatomic repair with transosseous fixation. In the experimental group (n = 24), diabetes was induced preoperatively via intraperitoneal injection of streptozotocin (STZ, 65 mg/kg) and confirmed with both pre- and post-STZ injection intraperitoneal glucose tolerance tests (IPGTT). Animals were sacrificed at 1 and 2 weeks postoperatively for biomechanical, histomorphometric, and immunohistochemical analysis. Serum hemoglobin A1c (HbA1c) levels were measured at 2 weeks postoperatively. Statistical comparisons were performed using Student t tests with significance set at P < .05.
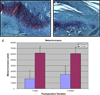
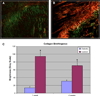
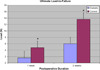
Results: IPGTT analysis demonstrated a significant impairment of glycemic control in the diabetic compared to control animals (P < .05). Mean HbA1c level at 2 weeks postoperatively was 10.6 ± 2.7% and 6.0 ± 1.0% for the diabetic and control groups, respectively (P < .05). Diabetic animals demonstrated significantly less fibrocartilage and organized collagen, and increased AGE deposition at the tendon-bone interface (P < .05). The healing enthesis of diabetic animals demonstrated a significantly reduced ultimate load-to-failure (4.79 ± 1.33 N vs 1.60 ± 1.67 N and 13.63 ± 2.33 N vs 6.0 ± 3.24 N for control versus diabetic animals at 1 and 2 weeks, respectively) and stiffness compared to control animals (P < .05).
Discussion: Sustained hyperglycemia impairs tendon-bone healing after rotator cuff repair in this rodent model. These findings have significant clinical implications for the expected outcomes of soft tissue repair or reconstructive procedures in diabetic patients with poor glycemic control.
Copyright © 2010 Journal of Shoulder and Elbow Surgery Board of Trustees. Published by Mosby, Inc. All rights reserved.
Conflict of interest statement
Disclaimer The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article and have no potential conflicts of interest related to this manuscript.
Figures

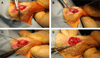


Similar articles
-
The effect of matrix metalloproteinase inhibition on tendon-to-bone healing in a rotator cuff repair model.J Shoulder Elbow Surg. 2010 Apr;19(3):384-91. doi: 10.1016/j.jse.2009.07.010. Epub 2009 Oct 2. J Shoulder Elbow Surg. 2010. PMID: 19800260
-
Is a Local Administration of Parathyroid Hormone Effective to Tendon-to-Bone Healing in a Rat Rotator Cuff Repair Model?J Orthop Res. 2020 Jan;38(1):82-91. doi: 10.1002/jor.24452. Epub 2019 Aug 29. J Orthop Res. 2020. PMID: 31441073
-
Calcium-phosphate matrix with or without TGF-β3 improves tendon-bone healing after rotator cuff repair.Am J Sports Med. 2011 Apr;39(4):811-9. doi: 10.1177/0363546511399378. Epub 2011 Mar 15. Am J Sports Med. 2011. PMID: 21406666
-
Current research trends on the effect of diabetes mellitus on rotator cuff tendon healing/tendinopathy.Arch Orthop Trauma Surg. 2024 Jun;144(6):2491-2500. doi: 10.1007/s00402-024-05350-1. Epub 2024 May 2. Arch Orthop Trauma Surg. 2024. PMID: 38698293 Review.
-
Growth and mechanobiology of the tendon-bone enthesis.Semin Cell Dev Biol. 2022 Mar;123:64-73. doi: 10.1016/j.semcdb.2021.07.015. Epub 2021 Aug 3. Semin Cell Dev Biol. 2022. PMID: 34362655 Free PMC article. Review.
Cited by
-
Risk Factors, Incidence, and Management of Re-Injury following Repair of Shoulder Rotator Cuff.J Orthop Sports Med. 2025;7(1):179-185. doi: 10.26502/josm.511500193. Epub 2025 Mar 31. J Orthop Sports Med. 2025. PMID: 40303933 Free PMC article.
-
Clinical and Structural Outcomes After Rotator Cuff Repair in Patients With Diabetes: A Meta-analysis.Orthop J Sports Med. 2020 Sep 17;8(9):2325967120948499. doi: 10.1177/2325967120948499. eCollection 2020 Sep. Orthop J Sports Med. 2020. PMID: 32995347 Free PMC article. Review.
-
Arthritis Severity and Medical Comorbidities Are Prognostic of Worse Outcomes Following Arthroscopic Rotator Cuff Repair in Patients With Concomitant Glenohumeral Osteoarthritis.Arthrosc Sports Med Rehabil. 2022 Oct 6;4(6):e1969-e1977. doi: 10.1016/j.asmr.2022.08.005. eCollection 2022 Dec. Arthrosc Sports Med Rehabil. 2022. PMID: 36579053 Free PMC article.
-
Obesity/Type II diabetes alters macrophage polarization resulting in a fibrotic tendon healing response.PLoS One. 2017 Jul 7;12(7):e0181127. doi: 10.1371/journal.pone.0181127. eCollection 2017. PLoS One. 2017. PMID: 28686669 Free PMC article.
-
Use of biologics in rotator cuff disorders: Current concept review.J Clin Orthop Trauma. 2021 May 15;19:81-88. doi: 10.1016/j.jcot.2021.05.005. eCollection 2021 Aug. J Clin Orthop Trauma. 2021. PMID: 34099971 Free PMC article.
References
-
- Akturk M, Karaahmetoglu S, Kacar M, Muftuoglu O. Thickness of the supraspinatus and biceps tendons in diabetic patients. Diabetes Care. 2002;25:408. - PubMed
-
- Allen DW, Schroeder WA, Balog J. Observations on the chromatographic heterogeneity of normal adult and fetal human hemoglobin: A study on the effects of crystallization and chromatography on the heterogeneity and isoleucine content. J Am Chem Soc. 1958;80:1628–1634.
-
- Alt N, Carson JA, Alderson NL, Wanh Y, Nagai R, Henle T, et al. Chemical modification of muscle protein in diabetes. Arch Biochem Biophys. 2004;425:200–206. - PubMed
-
- Altinel L, Cagri Kose K, Degirmenci B, Petik B, Acarturk G, Colbay M. The midterm effect of diabetes mellitus on quadriceps and patellar tendons in patients with knee arthrosis: a comparative radiologic study. J Diabetes Compl. 2007;21:392–396. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

