Privileged scaffolds for library design and drug discovery
- PMID: 20303320
- PMCID: PMC2908274
- DOI: 10.1016/j.cbpa.2010.02.018
Privileged scaffolds for library design and drug discovery
Abstract
This review explores the concept of using privileged scaffolds to identify biologically active compounds through building chemical libraries. We hope to accomplish three main objectives: to provide one of the most comprehensive listings of privileged scaffolds; to reveal through four selected examples the present state of the art in privileged scaffold library synthesis (in hopes of inspiring new and even more creative approaches); and also to offer some thoughts on how new privileged scaffolds might be identified and exploited.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

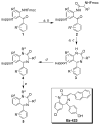
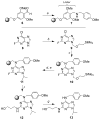
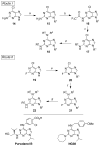
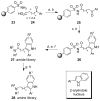
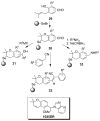
References
-
- Payne DJ, Gwynn MN, Holmes DJ, Pomplinano DL. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nature Reviews Drug Discovery. 2007;6:29–40. - PubMed
-
- Breinbauer R, Vetter IR, Waldmann H. From protein domains to drug candidates-natural products as guiding principles in the design and synthesis of compound libraries. Angew Chem Int Ed. 2002;41:2878–2890. The authors discuss natural products as a biologically validated starting point for library construction. They analyze rational library design in the context of structural biology and bioinformatics. - PubMed
-
- Evans BE, Rittle KE, Bock MG, DiPardo RM, Freidinger RM, Whitter WL, Lundell GF, Veber DF, Anderson PS, Chang RSL, Lotti VJ, Cerino DJ, Chen TB, Kling PJ, Kunkel KA, Springer JP, Hirshfield J. Methods for drug discovery: development of potent, selective, orally effective cholecystokinin antagonists. J Med Chem. 1988;31:2235–2246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

