Intra-myocardial delivery of mesenchymal stem cells ameliorates left ventricular and cardiomyocyte contractile dysfunction following myocardial infarction
- PMID: 20303399
- PMCID: PMC2862825
- DOI: 10.1016/j.toxlet.2010.03.009
Intra-myocardial delivery of mesenchymal stem cells ameliorates left ventricular and cardiomyocyte contractile dysfunction following myocardial infarction
Abstract
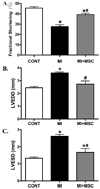
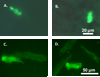
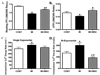
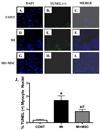
Although mesenchymal stem cells (MSCs) transplantation may improve the overall heart function, the heterogeneity of myocardial cells makes it difficult to determine the nature of cells benefited from transplantation. This study evaluated the effect of intra-myocardial MSC transplantation on myocardial function following MI. Enhanced green fluorescent protein (EGFP)-expressing donor MSCs from C57BL/6-Tg (UBC-GFP) 30Scha/J mice were transplanted into LV free wall in the region bordering an infarct in C57 recipient mice following ligation of left main coronary artery (MI+MSC group). Ten days after MI, LV function was assessed using echocardiography. Cardiomyocyte contractility and intracellular Ca(2+) transients were measured in cells from the area-at-risk surrounding the infarct scar. The EGFP donor cells were traced in the MSC recipient mice using fluorescence microscopy. TUNEL, H&E and Masson trichrome staining were used to assess apoptosis, angiogenesis and myocardial fibrosis, respectively. MI dilated LV as evidenced by increased end-diastolic and end-systolic diameters. MI significantly reduced fractional shortening, cardiomyocyte peak shortening, and maximal velocity of shortening and relengthening, all of which were attenuated or abrogated by MSC therapy. MI also reduced resting intracellular Ca(2+), intracellular Ca(2+) rise and decay rate, which were reconciled by MSC. MSC therapy attenuated MI-induced apoptosis and decreased angiogenesis but not myocardial fibrosis in the peri-infarct area. Taken together, our results demonstrated that MSC therapy significantly improved both LV and cardiomyocyte function possibly associated with its beneficial role in apoptosis and angiogenesis, indicating a key role for cardiomyocytes in stem cell tissue engineering.
Copyright 2010 Elsevier Ireland Ltd. All rights reserved.
Figures


Similar articles
-
Beclin1 haploinsufficiency compromises mesenchymal stem cell-offered cardioprotection against myocardial infarction.Cell Regen. 2022 Jun 2;11(1):21. doi: 10.1186/s13619-022-00121-y. Cell Regen. 2022. PMID: 35650374 Free PMC article.
-
Mesenchymal stem cell therapy associated with endurance exercise training: Effects on the structural and functional remodeling of infarcted rat hearts.J Mol Cell Cardiol. 2016 Jan;90:111-9. doi: 10.1016/j.yjmcc.2015.12.012. Epub 2015 Dec 15. J Mol Cell Cardiol. 2016. PMID: 26705058
-
Functional and bioenergetic modulations in the infarct border zone following autologous mesenchymal stem cell transplantation.Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1772-80. doi: 10.1152/ajpheart.00242.2007. Epub 2007 Jun 15. Am J Physiol Heart Circ Physiol. 2007. PMID: 17573463
-
Mesenchymal stem cell transplantation improves regional cardiac remodeling following ovine infarction.Stem Cells Transl Med. 2012 Sep;1(9):685-95. doi: 10.5966/sctm.2012-0027. Epub 2012 Sep 7. Stem Cells Transl Med. 2012. PMID: 23197875 Free PMC article.
-
Clinical aspects of left ventricular diastolic function assessed by Doppler echocardiography following acute myocardial infarction.Dan Med Bull. 2001 Nov;48(4):199-210. Dan Med Bull. 2001. PMID: 11767125 Review.
Cited by
-
Comparison of cardiac stem cells and mesenchymal stem cells transplantation on the cardiac electrophysiology in rats with myocardial infarction.Stem Cell Rev Rep. 2013 Jun;9(3):339-49. doi: 10.1007/s12015-012-9367-6. Stem Cell Rev Rep. 2013. PMID: 22544360
-
Placenta-Derived Adherent Stromal Cells Improve Diabetes Mellitus-Associated Left Ventricular Diastolic Performance.Stem Cells Transl Med. 2017 Dec;6(12):2135-2145. doi: 10.1002/sctm.17-0130. Epub 2017 Oct 10. Stem Cells Transl Med. 2017. PMID: 29024485 Free PMC article.
-
Oxidative activation of Ca(2+)/calmodulin-activated kinase II mediates ER stress-induced cardiac dysfunction and apoptosis.Am J Physiol Heart Circ Physiol. 2013 Mar 15;304(6):H828-39. doi: 10.1152/ajpheart.00752.2012. Epub 2013 Jan 11. Am J Physiol Heart Circ Physiol. 2013. PMID: 23316062 Free PMC article.
-
Therapeutic angiogenesis promotes efficacy of human umbilical cord matrix stem cell transplantation in cardiac repair.Iran J Basic Med Sci. 2015 Jun;18(6):563-70. Iran J Basic Med Sci. 2015. PMID: 26221480 Free PMC article.
-
Mesenchymal stem cells in cardiac regeneration: a detailed progress report of the last 6 years (2010-2015).Stem Cell Res Ther. 2016 Jun 4;7(1):82. doi: 10.1186/s13287-016-0341-0. Stem Cell Res Ther. 2016. PMID: 27259550 Free PMC article. Review.
References
-
- Anjos-Afonso F, Siapati EK, Bonnet D. In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. J. Cell Sci. 2004;117:5655–5664. - PubMed
-
- Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290:H2196–H2203. - PubMed
-
- Du YY, Zhou SH, Zhou T, Su H, Pan HW, Du WH, Liu B, Liu QM. Immuno-inflammatory regulation effect of mesenchymal stem cell transplantation in a rat model of myocardial infarction. Cytotherapy. 2008;10:469–478. - PubMed
-
- Fukuhara S, Tomita S, Nakatani T, Yutani C, Kitamura S. Endogenous bone-marrow-derived stem cells contribute only a small proportion of regenerated myocardium in the acute infarction model. J. Heart Lung Transplant. 2005;24:67–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

