Timing is everything: temporal processing deficits in the aged auditory brainstem
- PMID: 20303402
- PMCID: PMC7045868
- DOI: 10.1016/j.heares.2010.03.002
Timing is everything: temporal processing deficits in the aged auditory brainstem
Abstract
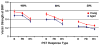
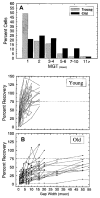
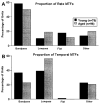
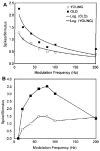
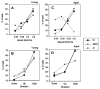
This summary article reviews the literature on neural correlates of age-related changes in temporal processing in the auditory brainstem. Two types of temporal processing dimensions are considered, (i) static, which can be measured using a gap detection or forward masking paradigms, and (ii) dynamic, which can be measured using amplitude and frequency modulation. Corresponding data from physiological studies comparing neural responses from young and old animals using acoustic stimuli as silent gaps-in-noise, amplitude modulation, and frequency modulation are considered in relation to speech perception. Evidence from numerous investigations indicates an age-related decline in encoding of temporal sound features which may be a contributing factor to the deficits observed in speech recognition in many elderly listeners.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures
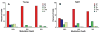

References
-
- Aitkin LM, Gates GR, et al. Responses of neurons in inferior colliculus to variations in sound-source azimuth. J Neurophysiol. 1984;52 (1):1–17. - PubMed
-
- Aitkin LM, Pettigrew JD, et al. Representation of stimulus azimuth by low-frequency neurons in inferior colliculus of the cat. J Neurophysiol. 1985;53 (1):43–59. - PubMed
-
- Aitkin L, Tran L, et al. The responses of neurons in subdivisions of the inferior colliculus of cats to tonal, noise and vocal stimuli. Exp Brain Res. 1994;98 (1):53–64. - PubMed
-
- Backoff PM, Shadduck Palombi P, et al. Glycinergic and GABAergic inputs affect short-term suppression in the cochlear nucleus. Hear Res. 1997;110 (1–2):155–163. - PubMed
-
- Backoff PM, Shadduck Palombi P, et al. Gamma-aminobutyric acidergic and glycinergic inputs shape coding of amplitude modulation in the chinchilla cochlear nucleus. Hear Res. 1999;134 (1–2):77–88. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

