Membrane rafts in Alzheimer's disease beta-amyloid production
- PMID: 20303415
- PMCID: PMC2886169
- DOI: 10.1016/j.bbalip.2010.03.007
Membrane rafts in Alzheimer's disease beta-amyloid production
Abstract
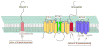
Alzheimer's disease (AD), the most common age-associated dementing disorder, is pathologically manifested by progressive cognitive dysfunction concomitant with the accumulation of senile plaques consisting of amyloid-beta (Abeta) peptide aggregates in the brain of affected individuals. Abeta is derived from a type I transmembrane protein, amyloid precursor protein (APP), by the sequential proteolytic events mediated by beta-site APP cleaving enzyme 1 (BACE1) and gamma-secretase. Multiple lines of evidence have implicated cholesterol and cholesterol-rich membrane microdomains, termed lipid rafts in the amyloidogenic processing of APP. In this review, we summarize the cell biology of APP, beta- and gamma-secretases and the data on their association with lipid rafts. Then, we will discuss potential raft targeting signals identified in the secretases and their importance on amyloidogenic processing of APP.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures

References
-
- Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. Allgemeine Zeitschrift für Psychiatrie und Psychisch-Gerichtliche Medizin. 1907;64:146–148.
-
- Vassar R. BACE1: the beta-secretase enzyme in Alzheimer’s disease. J Mol Neurosci. 2004;23:105–114. - PubMed
-
- Allinson TM, Parkin ET, Turner AJ, Hooper NM. ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res. 2003;74:342–352. - PubMed
-
- Iwatsubo T. The g-secretase complex: machinery for intramembrane proteolysis. Curr Opin Neurobiol. 2004;14:379–383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

