Nuclear factor-kappa B (NFkappaB) component p50 in blood mononuclear cells regulates endothelial tissue factor expression in sickle transgenic mice: implications for the coagulopathy of sickle cell disease
- PMID: 20303465
- PMCID: PMC2847430
- DOI: 10.1016/j.trsl.2009.10.004
Nuclear factor-kappa B (NFkappaB) component p50 in blood mononuclear cells regulates endothelial tissue factor expression in sickle transgenic mice: implications for the coagulopathy of sickle cell disease
Abstract
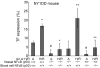
Sickle cell anemia is accompanied by the activation of coagulation and thrombosis. We have studied the abnormal expression of tissue factor (TF) by the pulmonary vein endothelium of the mild-phenotype NY1DD sickle transgenic. As detected by immunofluorescence microscopy, this occurs only after the NY1DD mouse is exposed to hypoxia/reoxygenation (H/R), which actually causes ischemia/reperfusion in the sickle cell disease-but not the normal-mouse model. We tested the hypothesis that the nuclear factor-kappa B (NFkappaB)-activating inflammation that develops in post-H/R NY1DD mice is responsible for this phenotype switch. Various NFkappaB inhibitors (including p50-specific andrographolide) demonstrated that endothelial TF positivity is NFkappaB dependent. Several systemic inflammatory stimulators (tumor necrosis factor [TNFalpha], lipopolysaccharide, thioglycollate, and carageenan) given to control mice showed that the inflammatory promotion of TF expression by only pulmonary vein endothelium is not specific to the sickle cell disease model. We bred the NFkappaB(p50)-/- state into the NY1DD mouse. Combined with marrow transplantation, this allowed the creation of NY1DD mice that were NFkappaB(p50)-/- only in peripheral blood cells (and marrow) versus only in vessel walls (and tissues). This process revealed that endothelial TF expression in the NY1DD mouse is highly dependent on NFkappaB(p50) in peripheral blood mononuclear cells-but not in the vessel wall. In confirmation, the infusion of post-H/R sickle mouse blood mononuclear cells into naïve NY1DD mice stimulated endothelial TF expression; the infusion of such cells from unstimulated sickle cell disease mice at ambient air did not stimulate TF expression. We conclude that peripheral blood mononuclear cells indirectly promote endothelial TF expression via a NFkappaB(p50)-dependent mechanism. This approach may be relevant to the role of coagulopathy in clinical sickle cell disease.
Copyright 2010 Mosby, Inc. All rights reserved.
Figures
References
-
- Francis RB, Jr, Hebbel RP. Hemostasis. In: Embury SH, Hebbel RP, Steinberg MH, Mohandas N, editors. Sickle Cell Disease: Basic Principles and Clinical Practice. New York: Raven Press; 1994. pp. 299–310.
-
- Tomer A, Harker LA, Kasey S, Eckman JR. Thrombogenesis in sickle cell disease. J Lab Clin Med. 2001;137:398–407. - PubMed
-
- Solovey A, Kollander R, Shet A, Milbauer LC, Choong S, Panoskaltsis-Mortari A, Blazar BR, Kelm RJ, Jr, Hebbel RP. Endothelial cell expression of tissue factor in sickle mice is augmented by hypoxia/reoxygenation and inhibited by lovastatin. Blood. 2004;104:840–846. - PubMed
-
- Osarogiagbon UR, Choong S, Belcher JD, Vercellotti GM, Paller MS, Hebbel RP. Reperfusion injury pathophysiology in sickle transgenic mice. Blood. 2000;96:314–20. - PubMed
-
- Kalambur VS, Mahaseh H, Bischof JC, Keilbik MC, Welch TE, Viback A, Swanlund DR, Hebbel RP, Belcher JD, Vercellotti GM. Microvascular blood flow and stasis in transgenic sickle mice: Utility of a dorsal skin fold chamber for intravital microscopy. Am J Hematol. 2004;77:117–125. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

