Tg.2098 is a major human thyroglobulin T-cell epitope
- PMID: 20303712
- PMCID: PMC2878895
- DOI: 10.1016/j.jaut.2010.01.004
Tg.2098 is a major human thyroglobulin T-cell epitope
Abstract
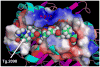
An HLA-DR variant containing Arginine at position 74 of the DRbeta1 chain confers a strong genetic susceptibility to autoimmune thyroid diseases (AITD), Graves' disease (GD) and Hashimoto's thyroiditis (HT), while Glutamine at position DRbeta1-74 is protective. We hypothesized that the DRbeta1-Arg74 variant is able to present pathogenic thyroglobulin (Tg) peptides to T-cells more efficiently, thereby triggering thyroid autoimmunity. Indeed, we have previously identified 4 human Tg (hTg) peptides that bind specifically to DRbeta1-Arg74 with much weaker binding to the protective variant DRbeta1-Gln74. The aim of our study was to examine in vivo whether an hTg peptide that binds strongly and specifically to DRbeta1-Arg74 is capable of stimulating T-cells during the induction of thyroiditis in a "humanized" mouse expressing human DR3, and in patients positive for Tg antibodies. Sequencing of exon 2 of the DR transgene in the DR3 mice, null for endogenous MHC II molecules, confirmed that they expressed the disease-associated DRbeta1-Arg74 variant, thus making them an ideal in vivo model to test the presentation of hTg peptides by DRbeta1-Arg74 HLA-DR. Induction of EAT in the DR3 mice lead to T-cell stimulation and proliferation to Tg.2098, a strong and specific DRbeta1-Arg74 binder, while a non-binding control peptide, Tg.2766 did not induce this response. Moreover, Tg.2098 stimulated T-cells from 4 individuals who were positive for thyroglobulin antibodies, demonstrating that Tg.2098 is an immunogenic peptide capable of being presented in vivo and activating T-cells in EAT and AITD. Energetic analysis of the complex formed by Tg.2098 and DRbeta-Arg74 has shown that the origin of the affinity was determined by residues 1, 7 and 9 in the peptide, while the selectivity of the peptide for the MHC was determined by the Asp in position 4. The disease-protective substitution R74Q, leads to reduction in affinity due to changes in local interaction with D4 as well as non-local interaction with other residues. The electrostatic potential on the surface of the DRbeta-Arg74-Tg.2098 complex has a unique signature which may be recognized by T-cell receptors leading to autoimmune thyroiditis. Taken together these findings suggest that Tg.2098, a strong and specific binder to the disease-associated HLA-DRbeta-Arg74, is a major human T-cell epitope and participant in the pathoetiology of AITD.
Figures






References
-
- Castellino F, Zhong G, Germain RN. Antigen presentation by MHC class II molecules: invariant chain function, protein trafficking, and the molecular basis of diverse determinant capture. Hum Immunol. 1997;54(2):159–169. - PubMed
-
- Lee KH, Wucherpfennig KW, Wiley DC. Structure of a human insulin peptide-HLA-DQ8 complex and susceptibility to type 1 diabetes. Nat Immunol. 2001;2(6):501–507. - PubMed
-
- Wucherpfennig KW. MHC-linked susceptibility to type 1 diabetes: a structural perspective. Ann N Y Acad Sci. 2003;1005:119–127. - PubMed
-
- Onengut-Gumuscu S, Concannon P. The genetics of type 1 diabetes: lessons learned and future challenges. J Autoimmun. 2005;25(Suppl):34–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

