N-acetylglutamate synthase: structure, function and defects
- PMID: 20303810
- PMCID: PMC2876818
- DOI: 10.1016/j.ymgme.2010.02.018
N-acetylglutamate synthase: structure, function and defects
Abstract
N-acetylglutamate (NAG) is a unique enzyme cofactor, essential for liver ureagenesis in mammals while it is the first committed substrate for de novo arginine biosynthesis in microorganisms and plants. The enzyme that produces NAG from glutamate and CoA, NAG synthase (NAGS), is allosterically inhibited by arginine in microorganisms and plants and activated in mammals. This transition of the allosteric effect occurred when tetrapods moved from sea to land. The first mammalian NAGS gene (from mouse) was cloned in 2002 and revealed significant differences from the NAGS ortholog in microorganisms. Almost all NAGS genes possess a C-terminus transferase domain in which the catalytic activity resides and an N-terminus kinase domain where arginine binds. The three-dimensional structure of NAGS shows two distinctly folded domains. The kinase domain binds arginine while the acetyltransferase domain contains the catalytic site. NAGS deficiency in humans leads to hyperammonemia and can be primary, due to mutations in the NAGS gene or secondary due to other mitochondrial aberrations that interfere with the normal function of the same enzyme. For either condition, N-carbamylglutamate (NCG), a stable functional analog of NAG, was found to either restore or improve the deficient urea-cycle function.
Copyright 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
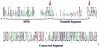



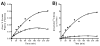
References
-
- Hall LM, Metzenberg RL, Cohen PP. Isolation and characterization of a naturally occurring cofactor of carbamyl phosphate biosynthesis. J Biol Chem. 1958;230:1013–1021. - PubMed
-
- Shigesada K, Tatibana M. Enzymatic synthesis of acetylglutamate by mammalian liver preparations and its stimulation by arginine. Biochem Biophys Res Commun. 1971;44:1117–1124. - PubMed
-
- Meijer AJ, Lamers WH, Chamuleau RA. Nitrogen metabolism and ornithine cycle function. Physiol Rev. 1990;70:701–748. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01DK53761/DK/NIDDK NIH HHS/United States
- P30 HD040677/HD/NICHD NIH HHS/United States
- K01DK076846/DK/NIDDK NIH HHS/United States
- R01 DK053761/DK/NIDDK NIH HHS/United States
- R01DK47870/DK/NIDDK NIH HHS/United States
- R01 HD058567/HD/NICHD NIH HHS/United States
- P30HD40677/HD/NICHD NIH HHS/United States
- K01 DK067935/DK/NIDDK NIH HHS/United States
- M01RR020359/RR/NCRR NIH HHS/United States
- P30HD2697/HD/NICHD NIH HHS/United States
- M01 RR020359/RR/NCRR NIH HHS/United States
- R01 DK064913/DK/NIDDK NIH HHS/United States
- K01 DK076846/DK/NIDDK NIH HHS/United States
- K01DK067935/DK/NIDDK NIH HHS/United States
- U54HD061221/HD/NICHD NIH HHS/United States
- R01 DK047870/DK/NIDDK NIH HHS/United States
- R01HD058567/HD/NICHD NIH HHS/United States
- U54RR019453/RR/NCRR NIH HHS/United States
- U54 HD061221/HD/NICHD NIH HHS/United States
- R01DK064913/DK/NIDDK NIH HHS/United States
- U54 RR019453/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

