Interaction of 18-methoxycoronaridine with nicotinic acetylcholine receptors in different conformational states
- PMID: 20303928
- PMCID: PMC3787694
- DOI: 10.1016/j.bbamem.2010.03.013
Interaction of 18-methoxycoronaridine with nicotinic acetylcholine receptors in different conformational states
Abstract
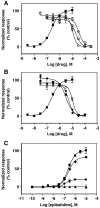
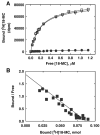
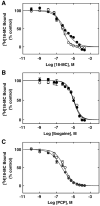
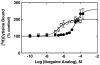
The interaction of 18-methoxycoronaridine (18-MC) with nicotinic acetylcholine receptors (AChRs) was compared with that for ibogaine and phencyclidine (PCP). The results established that 18-MC: (a) is more potent than ibogaine and PCP inhibiting (+/-)-epibatidine-induced AChR Ca(2+) influx. The potency of 18-MC is increased after longer pre-incubation periods, which is in agreement with the enhancement of [(3)H]cytisine binding to resting but activatable Torpedo AChRs, (b) binds to a single site in the Torpedo AChR with high affinity and inhibits [(3)H]TCP binding to desensitized AChRs in a steric fashion, suggesting the existence of overlapping sites. This is supported by our docking results indicating that 18-MC interacts with a domain located between the serine (position 6') and valine (position 13') rings, and (c) inhibits [(3)H]TCP, [(3)H]ibogaine, and [(3)H]18-MC binding to desensitized AChRs with higher affinity compared to resting AChRs. This can be partially attributed to a slower dissociation rate from the desensitized AChR compared to that from the resting AChR. The enthalpic contribution is more important than the entropic contribution when 18-MC binds to the desensitized AChR compared to that for the resting AChR, and vice versa. Ibogaine analogs inhibit the AChR by interacting with a luminal domain that is shared with PCP, and by inducing desensitization.
Figures



Similar articles
-
Structure-activity relationship of ibogaine analogs interacting with nicotinic acetylcholine receptors in different conformational states.Int J Biochem Cell Biol. 2011 Sep;43(9):1330-9. doi: 10.1016/j.biocel.2011.05.011. Epub 2011 May 27. Int J Biochem Cell Biol. 2011. PMID: 21642011
-
Interaction of ibogaine with human alpha3beta4-nicotinic acetylcholine receptors in different conformational states.Int J Biochem Cell Biol. 2010 Sep;42(9):1525-35. doi: 10.1016/j.biocel.2010.05.011. Int J Biochem Cell Biol. 2010. PMID: 20684041 Free PMC article.
-
Catharanthine alkaloids are noncompetitive antagonists of muscle-type nicotinic acetylcholine receptors.Neurochem Int. 2010 Sep;57(2):153-61. doi: 10.1016/j.neuint.2010.05.007. Epub 2010 May 20. Neurochem Int. 2010. PMID: 20493225
-
Binding sites for exogenous and endogenous non-competitive inhibitors of the nicotinic acetylcholine receptor.Biochim Biophys Acta. 1998 Aug 21;1376(2):173-220. doi: 10.1016/s0304-4157(98)00004-5. Biochim Biophys Acta. 1998. PMID: 9748559 Review.
-
Localization of agonist and competitive antagonist binding sites on nicotinic acetylcholine receptors.Neurochem Int. 2000 Jun;36(7):595-645. doi: 10.1016/s0197-0186(99)00154-0. Neurochem Int. 2000. PMID: 10771117 Review.
Cited by
-
Thyroid Peroxidase Activity is Inhibited by Phenolic Compounds-Impact of Interaction.Molecules. 2019 Jul 30;24(15):2766. doi: 10.3390/molecules24152766. Molecules. 2019. PMID: 31366075 Free PMC article.
-
Selective α3β4 Nicotinic Acetylcholine Receptor Ligand as a Potential Tracer for Drug Addiction.Int J Mol Sci. 2023 Feb 10;24(4):3614. doi: 10.3390/ijms24043614. Int J Mol Sci. 2023. PMID: 36835028 Free PMC article.
-
Preferential Coupling of Dopamine D2S and D2L Receptor Isoforms with Gi1 and Gi2 Proteins-In Silico Study.Int J Mol Sci. 2020 Jan 9;21(2):436. doi: 10.3390/ijms21020436. Int J Mol Sci. 2020. PMID: 31936673 Free PMC article.
-
Discovery of a potent and selective α3β4 nicotinic acetylcholine receptor antagonist from an α-conotoxin synthetic combinatorial library.J Med Chem. 2014 Apr 24;57(8):3511-21. doi: 10.1021/jm500183r. Epub 2014 Apr 3. J Med Chem. 2014. PMID: 24649848 Free PMC article.
-
Selectivity of coronaridine congeners at nicotinic acetylcholine receptors and inhibitory activity on mouse medial habenula.Int J Biochem Cell Biol. 2017 Nov;92:202-209. doi: 10.1016/j.biocel.2017.10.006. Epub 2017 Oct 16. Int J Biochem Cell Biol. 2017. PMID: 29042244 Free PMC article.
References
-
- Arias HR. Thermodynamics of nicotinic receptor interactions. In: Raffa RB, editor. Drug-Receptor Thermodynamics: Introduction and Applications. John Wiley & Sons, Ltd; USA: 2001. pp. 293–358.
-
- Arias HR. Ligand-gated ion channel receptor superfamilies. In: Arias HR, editor. Biological and Biophysical Aspects of Ligand-Gated Ion Channel Receptor Superfamilies. Research Signpost; Kerala, India: 2006. pp. 1–25.
-
- Arias HR, Bhumireddy P, Bouzat C. Molecular mechanisms and binding site locations for noncompetitive antagonists of nicotinic acetylcholine receptors. Int J Biochem Cell Biol. 2006;38:1254–1276. - PubMed
-
- Sine SM, Engel AG. Recent advances in Cys-loop receptor structure and function. Nature. 2006;440:448–455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

