Estrogen effects on pain sensitivity and neuropeptide expression in rat sensory neurons
- PMID: 20303952
- PMCID: PMC2885587
- DOI: 10.1016/j.expneurol.2010.03.006
Estrogen effects on pain sensitivity and neuropeptide expression in rat sensory neurons
Abstract
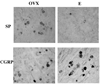
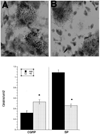
While a number of chronic pain conditions are much more prevalent in women than men, the role of estrogen in regulating nociception remains unclear. Estrogen receptors (ER) are known to be expressed in various parts of the nociceptive pathway, including in the small-sized primary sensory neurons of the dorsal root ganglion (DRG). This study evaluated the effects of long term estrogen replacement on pain sensitivity and neuropeptide expression in the DRG of female Sprague Dawley rats. The goal was to evaluate whether estrogen modulates nociceptive neuropeptides in the DRG in a manner consistent with its effects on pain sensitivity. Our results show that long term (28 days) ovariectomy (ovx) of adult rats induces a profound thermal and mechanical hyperalgesia of the hindpaw and tail compared to ovariectomized animals that were continuously estrogen-treated (ovx+E). Significant changes in the expression of two neuropeptides, substance P (SP) and calcitonin gene-related peptide (CGRP), were observed using immunocytochemistry and in situ hybridization (ISH) in the small lumbar DRG neurons which contain ER. CGRP and SP were differentially regulated by estrogen, with SP showing a significant downregulation at both the peptide and mRNA levels while CGRP and its mRNA were increased in the DRG of estrogen-treated animals. We also evaluated the development of mechanical allodynia after partial sciatic nerve injury and found that both ovx and ovx+E animals developed significant allodynia within a week of the partial nerve injury, which continued for at least one month. The estrogen-treated animals showed a partial amelioration of the extent of the allodynia at 2 weeks post injury. Overall, the results suggest that estrogen has significant anti-nociceptive actions that can be directly correlated with changes in expression of two peptides in the small nociceptive ERalpha expressing neurons of the DRG.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Substance P in the uterine cervix, dorsal root ganglia and spinal cord during pregnancy and the effect of estrogen on SP synthesis.Peptides. 2003 May;24(5):761-71. doi: 10.1016/s0196-9781(03)00120-7. Peptides. 2003. PMID: 12895664
-
Effect of Mas-related gene (Mrg) receptors on hyperalgesia in rats with CFA-induced inflammation via direct and indirect mechanisms.Br J Pharmacol. 2013 Nov;170(5):1027-40. doi: 10.1111/bph.12326. Br J Pharmacol. 2013. PMID: 23909597 Free PMC article.
-
The effects of pregnancy and estrogen on the expression of calcitonin gene-related peptide (CGRP) in the uterine cervix, dorsal root ganglia and spinal cord.Peptides. 2003 Aug;24(8):1163-74. doi: 10.1016/j.peptides.2003.07.009. Peptides. 2003. PMID: 14612187
-
Up-regulation of dorsal root ganglia BDNF and trkB receptor in inflammatory pain: an in vivo and in vitro study.J Neuroinflammation. 2011 Sep 30;8:126. doi: 10.1186/1742-2094-8-126. J Neuroinflammation. 2011. PMID: 21958434 Free PMC article.
-
Differential expression of estrogen receptor alpha and beta in rat dorsal root ganglion neurons.J Neurosci Res. 1999 Sep 1;57(5):603-15. J Neurosci Res. 1999. PMID: 10462685
Cited by
-
The influence of the sensory neurotransmitter calcitonin gene-related peptide on bone marrow mesenchymal stem cells from ovariectomized rats.J Bone Miner Metab. 2017 Sep;35(5):473-484. doi: 10.1007/s00774-016-0780-9. Epub 2016 Sep 13. J Bone Miner Metab. 2017. PMID: 27623790
-
Estrogens as arbiters of sex-specific and reproductive cycle-dependent opioid analgesic mechanisms.Vitam Horm. 2019;111:227-246. doi: 10.1016/bs.vh.2019.06.002. Epub 2019 Jul 2. Vitam Horm. 2019. PMID: 31421702 Free PMC article.
-
Estrogens synthesized and acting within a spinal oligomer suppress spinal endomorphin 2 antinociception: ebb and flow over the rat reproductive cycle.Pain. 2017 Oct;158(10):1903-1914. doi: 10.1097/j.pain.0000000000000991. Pain. 2017. PMID: 28902684 Free PMC article.
-
Neuropathic pain impairs sleep architecture, non-rapid eye movement sleep, and reticular thalamic neuronal activity.Int J Neuropsychopharmacol. 2025 May 9;28(5):pyaf017. doi: 10.1093/ijnp/pyaf017. Int J Neuropsychopharmacol. 2025. PMID: 40121517 Free PMC article.
-
17β-Estradiol Exacerbated Experimental Occlusal Interference-Induced Chronic Masseter Hyperalgesia by Increasing the Neuronal Excitability and TRPV1 Function of Trigeminal Ganglion in Ovariectomized Rats.Int J Mol Sci. 2021 Jun 28;22(13):6945. doi: 10.3390/ijms22136945. Int J Mol Sci. 2021. PMID: 34203300 Free PMC article.
References
-
- Ahmed Y, Lin DL, et al. Effect of estrogen on urethral function and nerve regeneration following pudendal nerve crush in the female rat. J. Urol. 2006;175:1948–1952. - PubMed
-
- Amara SG, Arriza JL, et al. Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science. 1985;209:1094–1097. - PubMed
-
- Berkley K. Sex differences in pain. Behav Brain Sci. 1997;20:371–380. - PubMed
-
- Brown ER, Harlan RE, et al. Gonadal steroid regulation of substance P (SP)-encoding messenger ribonucleic acids in the rat anterior pituitary and hypothalamus. Endocrin. 1990;126:330–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

