Membrane-binding and enzymatic properties of RPE65
- PMID: 20304090
- PMCID: PMC2903629
- DOI: 10.1016/j.preteyeres.2010.03.002
Membrane-binding and enzymatic properties of RPE65
Abstract
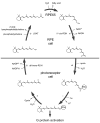
Regeneration of visual pigments is essential for sustained visual function. Although the requirement for non-photochemical regeneration of the visual chromophore, 11-cis-retinal, was recognized early on, it was only recently that the trans to cis retinoid isomerase activity required for this process was assigned to a specific protein, a microsomal membrane enzyme called RPE65. In this review, we outline progress that has been made in the functional characterization of RPE65. We then discuss general concepts related to protein-membrane interactions and the mechanism of the retinoid isomerization reaction and describe some of the important biochemical and structural features of RPE65 with respect to its membrane-binding and enzymatic properties.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

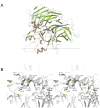
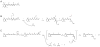

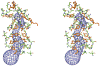
References
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4. Garland Science; New York: 2002.
-
- Balliano G, Viola F, Ceruti M, Cattel L. Characterization and partial purification of squalene-2,3-oxide cyclase from Saccharomyces cerevisiae. Arch Biochem Biophys. 1992;293:122–129. - PubMed
-
- Barry RJ, Canada FJ, Rando RR. Solubilization and partial purification of retinyl ester synthetase and retinoid isomerase from bovine ocular pigment epithelium. J Biol Chem. 1989;264:9231–9238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

