Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth
- PMID: 20304630
- PMCID: PMC2856784
- DOI: 10.1016/j.ejca.2010.02.027
Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth
Abstract
Metastases can develop after apparently successful treatment of a primary tumour, sometimes following a period of tumour dormancy that can last for years. However, factors that regulate metastatic tumour dormancy remain poorly understood. Here we review the potential contribution of interactions between tumour cells and the microenvironment in metastatic sites, in regulating tumour dormancy vs. metastatic growth. We focus particularly on the potential role of the extracellular matrix (ECM) in regulating maintenance and release from dormancy. Tumour cells that fail to properly adhere to the ECM may enter a state of dormancy. The molecular and physical composition of the ECM can be affected by tumour cells themselves, as well as multiple stromal cell types. The roles of integrins, fibronectin, and collagen are discussed, as are factors that can change the ECM. A better understanding of the molecular details of the crosstalk between tumour cells and the ECM in secondary sites, and how these regulate the dormant state, may lead to improved therapeutic strategies to induce or maintain disseminated tumour cells in a dormant state, or alternatively to successfully eradicate dormant cells.
Copyright (c) 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
None declared.
Figures

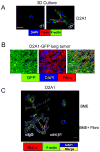
References
-
- Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4(6):448–56. - PubMed
-
- Naumov GN, MacDonald IC, Chambers AF, et al. Solitary cancer cells as a possible source of tumour dormancy? Semin Cancer Biol. 2001;11(4):271–6. - PubMed
-
- Townson JL, Chambers AF. Dormancy of solitary metastatic cells. Cell Cycle. 2006;(16):1744–50. - PubMed
-
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

