Synthesis and pharmacological characterization of a new series of 5,7-disubstituted-[1,2,4]triazolo[1,5-a][1,3,5]triazine derivatives as adenosine receptor antagonists: A preliminary inspection of ligand-receptor recognition process
- PMID: 20304654
- PMCID: PMC3106415
- DOI: 10.1016/j.bmc.2010.02.039
Synthesis and pharmacological characterization of a new series of 5,7-disubstituted-[1,2,4]triazolo[1,5-a][1,3,5]triazine derivatives as adenosine receptor antagonists: A preliminary inspection of ligand-receptor recognition process
Abstract
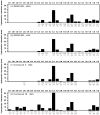
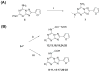
A new series of triazolotriazines variously substituted at the C5 and N7 (5-25) positions was synthesized and fully characterized at the four adenosine receptor (AR) subtypes. In particular, arylacetyl or arylcarbamoyl moieties were introduced at the N7 position, which enhanced affinity at the hA(2B) and hA(3) ARs, respectively, when utilized on the pyrazolo-triazolopyrimidine nucleus as we reported in the past. In general, compounds with a free amino group at the 7 position (5, 6), showed good affinity at the rat (r) A(2A) AR (range 18.3-96.5nM), while the introduction of a phenylcarbamoyl moiety at the N7 position (12, 19, 24) slightly increased the affinity at the hA(3) AR (range 311-633nM) with respect to the unsubstituted derivatives. The binding profiles of the synthesized analogues seemed to correlate with the substitutions at the C5 and N7 positions. At the hA(2B) AR, derivative 5, which contained a free amino group at the 7 position, was the most potent (EC(50) 3.42microM) and could represent a starting point for searching new non-xanthine hA(2B) AR antagonists. Molecular models of the rA(2A) and hA(3) ARs were constructed by homology to the recently reported crystallographic structure of the hA(2A) AR. A preliminary receptor-driven structure-activity relationship (SAR) based on the analysis of antagonist docking has been provided.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures


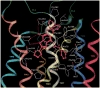

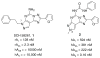
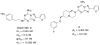


References
-
- Downey JM, Cohen MV, Ytrehus K, Liu Y. Ann NY Acad Sci. 1994;723:82. - PubMed
-
- Auchampach JA, Jin X, Wan TC, Caughey GH, Linden J. Mol Pharmacol. 1997;52:846. - PubMed
-
- Fredholm BB. Int Rev Neurobiol. 1997;40:259. - PubMed
-
- Feoktistov I, Polosa R, Holgate ST, Biaggioni I. Trends Pharmacol Sci. 1998;19:148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Research Materials
Miscellaneous

