Amyloid-like interactions within nucleoporin FG hydrogels
- PMID: 20304795
- PMCID: PMC2852002
- DOI: 10.1073/pnas.0910163107
Amyloid-like interactions within nucleoporin FG hydrogels
Abstract
The 62 kDa FG repeat domain of the nucleoporin Nsp1p forms a hydrogel-based, sieve-like permeability barrier that excludes inert macromolecules but allows rapid entry of nuclear transport receptors (NTRs). We found that the N-terminal part of this domain, which is characterized by Asn-rich inter-FG spacers, forms a tough hydrogel. The C-terminal part comprises charged inter-FG spacers, shows low gelation propensity on its own, but binds the N-terminal part and passivates the FG hydrogel against nonselective interactions. It was previously shown that a hydrophobic collapse involving Phe residues is required for FG hydrogel formation. Using solid-state NMR spectroscopy, we now identified two additional types of intragel interactions, namely, transient hydrophobic interactions between Phe and methyl side chains as well as intermolecular beta-sheets between the Asn-rich spacer regions. The latter appear to be the kinetically most stable structures within the FG hydrogel. They are also a central feature of neuronal inclusions formed by Asn/Gln-rich amyloid and prion proteins. The cohesive properties of FG repeats and the Asn/Gln-rich domain from the yeast prion Sup35p appear indeed so similar to each other that these two modules interact in trans. Our data, therefore, suggest a fully unexpected cellular function of such interchain beta-structures in maintaining the permeability barrier of nuclear pores. They provide an explanation for how contacts between FG repeats might gain the kinetic stability to suppress passive fluxes through nuclear pores and yet allow rapid NTR passage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
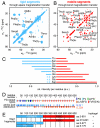
 domain. (E) Bar models of Nsp1p fragments with high and low gel-forming propensity as determined by qualitative gelation assays (
domain. (E) Bar models of Nsp1p fragments with high and low gel-forming propensity as determined by qualitative gelation assays (
 as compared to statistical average values for β-strands (green lines). (C) Dependence of Cα peak intensities for Asn, Thr, and Ser on the 1H-1H mixing time (tHH) in NHHC (25) experiments suggests NH-HCα proton-proton distances compatible with β-strands. (D) NHHC spectrum obtained for a hydrogel containing 13C-labeled
as compared to statistical average values for β-strands (green lines). (C) Dependence of Cα peak intensities for Asn, Thr, and Ser on the 1H-1H mixing time (tHH) in NHHC (25) experiments suggests NH-HCα proton-proton distances compatible with β-strands. (D) NHHC spectrum obtained for a hydrogel containing 13C-labeled  and 15N-labeled
and 15N-labeled  at a 1∶1 ratio. The signal arises due to short 1H-1H distances between complementary labeled β-strands forming an intermolecular sheet (see sketch and Materials and Methods for details).
at a 1∶1 ratio. The signal arises due to short 1H-1H distances between complementary labeled β-strands forming an intermolecular sheet (see sketch and Materials and Methods for details).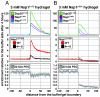
 ,
,  , and the N/Q-rich Sup35 prion domain specifically interact with Nsp1-derived hydrogels. (A) Hydrogel droplets containing 3 mM of the
, and the N/Q-rich Sup35 prion domain specifically interact with Nsp1-derived hydrogels. (A) Hydrogel droplets containing 3 mM of the  repeat domain were incubated with 2 μM of the indicated Atto488-labeled species (0.5 μM for Sup352-140). After 4 h, partitioning of the fluorescent species between buffer and gel phases was analyzed. Note that the full-length repeat domain (Nsp12-601) and the N-terminal portion with the highest gel-forming propensity (Nsp12-175) interacted strongly with the hydrogel. The prion domain of Sup35p (Sup352-140) showed an even higher partitioning into the gel phase. In comparison to a corresponding mutant repeat domain lacking all hydrophobic residues (Nsp1274-601 Φ→S) or a 20 kDa PEG polymer, also
repeat domain were incubated with 2 μM of the indicated Atto488-labeled species (0.5 μM for Sup352-140). After 4 h, partitioning of the fluorescent species between buffer and gel phases was analyzed. Note that the full-length repeat domain (Nsp12-601) and the N-terminal portion with the highest gel-forming propensity (Nsp12-175) interacted strongly with the hydrogel. The prion domain of Sup35p (Sup352-140) showed an even higher partitioning into the gel phase. In comparison to a corresponding mutant repeat domain lacking all hydrophobic residues (Nsp1274-601 Φ→S) or a 20 kDa PEG polymer, also  was considerably enriched within the
was considerably enriched within the  hydrogel. (B) Hydrogels with 3 mM
hydrogel. (B) Hydrogels with 3 mM  were analyzed exactly as in (A). In comparison to the
were analyzed exactly as in (A). In comparison to the  hydrogel, the inclusion of the
hydrogel, the inclusion of the  module into the gel not only blocked binding sites for fluorescent
module into the gel not only blocked binding sites for fluorescent  molecules (Middle), but also efficiently suppressed influx and partitioning of the inert PEG control polymer into the gel (Lower).
molecules (Middle), but also efficiently suppressed influx and partitioning of the inert PEG control polymer into the gel (Lower).
 hydrogel was about 3-fold slower than in the
hydrogel was about 3-fold slower than in the  hydrogel, while the enrichment of the NTR●cargo complexes at the buffer/gel boundary was increased by a factor of 2. The presence of the more C-terminal FxFG repeats harboring charged spacers also significantly reduced nonspecific binding of inert proteins to the hydrogels.
hydrogel, while the enrichment of the NTR●cargo complexes at the buffer/gel boundary was increased by a factor of 2. The presence of the more C-terminal FxFG repeats harboring charged spacers also significantly reduced nonspecific binding of inert proteins to the hydrogels.
 (as used for NMR).
(as used for NMR).
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

