Specific estrogen sulfotransferase (SULT1E1) substrates and molecular imaging probe candidates
- PMID: 20304798
- PMCID: PMC2852016
- DOI: 10.1073/pnas.0914904107
Specific estrogen sulfotransferase (SULT1E1) substrates and molecular imaging probe candidates
Abstract
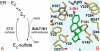
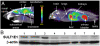
This work focuses on the development of specific substrates for estrogen sulfotransferase (SULT1E1) to produce molecular imaging probes for this enzyme. SULT1E1 is a key enzyme in estrogen homeostasis, playing a central role in the prevention and development of human disease. In vitro sulfation assays showed alkyl and aryl substitutions to a fused heterocyclic system modeled after beta-naphthol (betaN), based on compounds that interact with the estrogen receptor, rendered several molecules with enhanced specificity for SULT1E1 over SULT1A1*1, SULT1A1*2, SULT1A3, and SULT2A1. Several 6-hydroxy-2-arylbenzothiazoles tested demonstrated excellent affinity--V(max)/K(m) ratios-and specificity for SULT1E1. K(m) values ranged from 0.12-2.36 microM. A strong correlation was observed between polarity of the 4'-sustituent on the 2-aryl moiety (Hammett sigma(p)) and the log(V(max)/K(m)) (r = 0.964). Substrate sensitivity is influenced by the acidity of the 6-phenolic group demonstrated by correlating its (1)H NMR chemical shift (delta(OH)) with the log(V(max)/K(m)) (r = 0.963). Acidity is mediated by the electron withdrawing capacity of the 4'-substituent outlined by the correlation of the C-2 (13)C NMR chemical shift (delta(C2)) with the log(V(max)/K(m)) (r = 0.987). 2-[4-(Methylamino)phenyl]-6-hydroxybenzothiazole (2b) was radiolabeled with carbon-11 ((11)C-(2b)) and used in vivo for microPET scanning and tissue metabolite identification. High PET signal was paralleled with the presence of radiolabeled (11)C-(2b)-6-O-sulfate and the SULT1E1 protein detected by western blot. Because this and other members of this family presenting specificity for SULT1E1 can be labeled with carbon-11 or fluorine-18, in vivo assays of SULT1E1 functional activity are now feasible in humans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
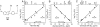


References
-
- Falany C. Enzymology of human cytosolic sulfotransferases. FASEB J. 1997;11(4):206–216. - PubMed
-
- Blanchard RL. Human cytosolic sulfotransferases. In: Pacifici GM, Coughtrie MWH, editors. Boca Raton, FL: Taylor and Francis Group, LLC; 2005. pp. 1–8.
-
- Miki Y, et al. Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fetal tissues. J Clin Endocrinol Metab. 2002;87(12):5760–5768. - PubMed
-
- Zhang H, Varmalova O, Vargas FM, Falany CN, Leyh TS. Sulfuryl transfer: The catalytic mechanism of human estrogen sulfotransferase. J Biol Chem. 1998;273(18):10888–10892. - PubMed
-
- Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun. 2005;328:688–696. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

