Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids
- PMID: 20304807
- PMCID: PMC2913454
- DOI: 10.1182/blood-2010-01-260372
Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids
Abstract
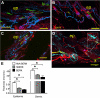
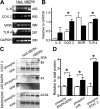
Sickle cell disease causes severe pain. We examined pain-related behaviors, correlative neurochemical changes, and analgesic effects of morphine and cannabinoids in transgenic mice expressing human sickle hemoglobin (HbS). Paw withdrawal threshold and withdrawal latency (to mechanical and thermal stimuli, respectively) and grip force were lower in homozygous and hemizygous Berkley mice (BERK and hBERK1, respectively) compared with control mice expressing human hemoglobin A (HbA-BERK), indicating deep/musculoskeletal and cutaneous hyperalgesia. Peripheral nerves and blood vessels were structurally altered in BERK and hBERK1 skin, with decreased expression of mu opioid receptor and increased calcitonin gene-related peptide and substance P immunoreactivity. Activators of neuropathic and inflammatory pain (p38 mitogen-activated protein kinase, STAT3, and mitogen-activated protein kinase/extracellular signal-regulated kinase) showed increased phosphorylation, with accompanying increase in COX-2, interleukin-6, and Toll-like receptor 4 in the spinal cord of hBERK1 compared with HbA-BERK. These neurochemical changes in the periphery and spinal cord may contribute to hyperalgesia in mice expressing HbS. In BERK and hBERK1, hyperalgesia was markedly attenuated by morphine and cannabinoid receptor agonist CP 55940. We show that mice expressing HbS exhibit characteristics of pain observed in sickle cell disease patients, and neurochemical changes suggestive of nociceptor and glial activation. Importantly, cannabinoids attenuate pain in mice expressing HbS.
Figures

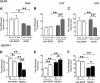


 , hBERK1 mice; and ■, BERK mice. *P < .01.
, hBERK1 mice; and ■, BERK mice. *P < .01.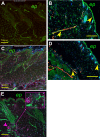
Comment in
-
New era dawns on sickle cell pain.Blood. 2010 Jul 22;116(3):311-2. doi: 10.1182/blood-2010-04-276386. Blood. 2010. PMID: 20651080 No abstract available.
References
-
- Ballas SK. Progress in Pain Research and Management. vol 11. Seattle WA: IASP Press; 1998.
-
- Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148(2):94–101. - PubMed
-
- Ballas SK. Current issues in sickle cell pain and its management. Hematology Am Soc Hematol Educ Program. 2007:97–105. - PubMed
-
- Dampier CD, Setty BN, Logan J, Ioli JG, Dean R. Intravenous morphine pharmacokinetics in pediatric patients with sickle cell disease. J Pediatr. 1995;126(3):461–467. - PubMed
-
- Darbari DS, Minniti CP, Rana S, van den Anker J. Pharmacogenetics of morphine: potential implications in sickle cell disease. Am J Hematol. 2008;83(3):233–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

