Clinical significance of low levels of minimal residual disease at the end of remission induction therapy in childhood acute lymphoblastic leukemia
- PMID: 20304809
- PMCID: PMC2890183
- DOI: 10.1182/blood-2009-11-253435
Clinical significance of low levels of minimal residual disease at the end of remission induction therapy in childhood acute lymphoblastic leukemia
Abstract
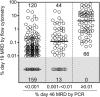
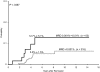
Minimal residual disease (MRD) at the end of remission-induction therapy predicts relapse in acute lymphoblastic leukemia (ALL). We examined the clinical significance of levels below the usual threshold value for MRD positivity (0.01%) in 455 children with B-lineage ALL, using polymerase chain reaction amplification of antigen-receptor genes capable of detecting at least 1 leukemic cell per 100 000 normal mononucleated cells (0.001%). Of the 455 clinical samples studied on day 46 of therapy, 139 (30.5%) had MRD 0.001% or more with 63 of these (45.3%) showing levels of 0.001% to less than 0.01%, whereas 316 (69.5%) had levels that were either less than 0.001% or undetectable. MRD measurements of 0.001% to less than 0.01% were not significantly related to presenting characteristics but were associated with a poorer leukemia cell clearance on day 19 of remission induction therapy. Patients with this low level of MRD had a 12.7% (+/- 5.1%; SE) cumulative risk of relapse at 5 years, compared with 5.0% (+/- 1.5%) for those with lower or undetectable MRD (P < .047). Thus, low levels of MRD (0.001%-< 0.01%) at the end of remission induction therapy have prognostic significance in childhood ALL, suggesting that patients with this finding should be monitored closely for adverse events.
Figures
References
-
- Möricke A, Reiter A, Zimmermann M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood. 2008;111(9):4477–4489. - PubMed
-
- Pui CH, Campana D, Evans WE. Childhood acute lymphoblastic leukemia: current status and future perspectives. Lancet Oncol. 2001;2(10):597–607. - PubMed
-
- Campana D. Molecular determinants of treatment response in acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2008;2008:366–373. - PubMed
-
- Cavé H, van der Werff ten Bosch J, Suciu S, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia: European Organization for Research and Treatment of Cancer–Childhood Leukemia Cooperative Group. N Engl J Med. 1998;339(9):591–598. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

