Cross talk between the bone and immune systems: osteoclasts function as antigen-presenting cells and activate CD4+ and CD8+ T cells
- PMID: 20304810
- PMCID: PMC2910608
- DOI: 10.1182/blood-2009-11-255026
Cross talk between the bone and immune systems: osteoclasts function as antigen-presenting cells and activate CD4+ and CD8+ T cells
Abstract
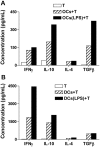
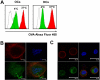
The bone and immune systems are closely related through cellular and molecular interactions. Because bone-resorbing osteoclasts (OCs) are derived from the monocyte/macrophage lineage, similar to dendritic cells (DCs), we hypothesized that OCs could serve as antigen-presenting cells (APCs) to activate T cells. In this study, OCs were generated from human monocytes with stimulation by receptor activator of nuclear factor kappaB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF). Results showed that, similar to DCs, OCs express major histocompatibility complex (MHC) classes I and II, and CD80, CD86, and CD40; and uptake soluble antigens. OCs secrete interleukin-10 (IL-10), transforming growth factor-beta (TGF-beta), IL-6, and tumor necrosis factor-alpha (TNF-alpha), but not IL-12p70. OCs present allogeneic antigens and activate both CD4+ and CD8+ alloreactive T cells in an MHC-restricted fashion. OCs also present soluble protein tetanus toxoid to activate autologous CD4+ T cells. These findings indicate that OCs can function as APCs and activate both CD4+ and CD8+ T cells. Thus, our study provides new insight into the effect of OCs on the immune system and may help develop novel strategies for treating diseases such as rheumatoid arthritis and multiple myeloma, which affect both the bone and immune systems.
Figures



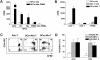

References
-
- Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002;8(3):147–159. - PubMed
-
- Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7(4):292–304. - PubMed
-
- Wong BR, Rho J, Arron J, et al. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem. 1997;272(40):25190–25194. - PubMed
-
- Wong BR, Josien R, Choi Y. TRANCE is a TNF family member that regulates dendritic cell and osteoclast function. J Leukoc Biol. 1999;65(6):715–724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

