MnSOD protects against COX1-mediated endothelial dysfunction in chronic heart failure
- PMID: 20304815
- PMCID: PMC2867433
- DOI: 10.1152/ajpheart.01108.2009
MnSOD protects against COX1-mediated endothelial dysfunction in chronic heart failure
Abstract
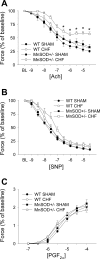
Endothelial function is impaired by oxidative stress in chronic heart failure (HF). Mechanisms that protect against increases in oxidative stress in HF are not clear. The goal of this study was to determine whether manganese superoxide dismutase (MnSOD) plays a key role in protecting against endothelial dysfunction in HF. Endothelial function and gene expression were examined in aorta from wild-type mice (MnSOD(+/+)) and mice deficient in MnSOD (MnSOD(+/-)) 12 wk after ligation of the left coronary artery (LCA). LCA ligation produced similar size myocardial infarctions in MnSOD(+/+) and MnSOD(+/-) mice and reduced ejection fraction to approximately 20% in both groups. Maximal relaxation in response to acetylcholine was 78 +/- 3% (mean +/- SE) and 66 +/- 8% in sham-operated MnSOD(+/+) and MnSOD(+/-) mice, respectively. Expression of antioxidant enzymes increased in MnSOD(+/+) mice with HF, and maximal relaxation to acetylcholine was slightly impaired (68 +/- 4%). Greater endothelial dysfunction was observed in MnSOD(+/-) mice with HF (46 +/- 5%, P < 0.05), which was significantly improved by polyethylene glycol-catalase but not Tempol. Incubation with the nonspecific cyclooxygenase (COX) inhibitor indomethacin or the COX1 inhibitor valeryl salicylate, but not the COX-2 inhibitor NS-398, significantly improved relaxation to acetylcholine in HF mice (maximum relaxation = 74 +/- 5, 91 +/- 1, and 58 +/- 5%). These data suggest that MnSOD plays a key role in protecting against endothelial dysfunction in HF. A novel mechanism was identified whereby chronic increases in oxidative stress, produced by mitochondrial SOD deficiency, impair vascular function via a hydrogen peroxide-dependent, COX1-dependent, endothelium-derived contracting factor.
Figures

Similar articles
-
Protective effect of extracellular superoxide dismutase on endothelial function during aging.Am J Physiol Heart Circ Physiol. 2009 Jun;296(6):H1920-5. doi: 10.1152/ajpheart.01342.2008. Epub 2009 Apr 17. Am J Physiol Heart Circ Physiol. 2009. PMID: 19376805 Free PMC article.
-
Sex differences in protection against angiotensin II-induced endothelial dysfunction by manganese superoxide dismutase in the cerebral circulation.Hypertension. 2010 Apr;55(4):905-10. doi: 10.1161/HYPERTENSIONAHA.109.147041. Epub 2010 Mar 1. Hypertension. 2010. PMID: 20194298 Free PMC article.
-
Aerobic exercise training protects against endothelial dysfunction by increasing nitric oxide and hydrogen peroxide production in LDL receptor-deficient mice.J Transl Med. 2016 Jul 19;14(1):213. doi: 10.1186/s12967-016-0972-z. J Transl Med. 2016. PMID: 27435231 Free PMC article.
-
Endothelial control of vascular and myocardial function in heart failure.Cardiovasc Drugs Ther. 1994 Jun;8(3):437-46. doi: 10.1007/BF00877920. Cardiovasc Drugs Ther. 1994. PMID: 7947359 Review.
-
Vascular protection: superoxide dismutase isoforms in the vessel wall.Arterioscler Thromb Vasc Biol. 2004 Aug;24(8):1367-73. doi: 10.1161/01.ATV.0000133604.20182.cf. Epub 2004 May 27. Arterioscler Thromb Vasc Biol. 2004. PMID: 15166009 Review.
Cited by
-
Impact of neonatal sertraline exposure on the post-myocardial infarction outcomes of adult male mice.J Cardiovasc Pharmacol. 2013 Nov;62(5):479-84. doi: 10.1097/FJC.0b013e3182a4db90. J Cardiovasc Pharmacol. 2013. PMID: 23921310 Free PMC article.
-
Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice.Aging Cell. 2016 Oct;15(5):973-7. doi: 10.1111/acel.12458. Epub 2016 Aug 5. Aging Cell. 2016. PMID: 26864908 Free PMC article.
-
Vascular endothelial function is not related to serum uric acid in healthy adults.Am J Hypertens. 2012 Apr;25(4):407-13. doi: 10.1038/ajh.2011.237. Epub 2012 Jan 12. Am J Hypertens. 2012. PMID: 22237152 Free PMC article.
-
Mitochondria, endothelial cell function, and vascular diseases.Front Physiol. 2014 May 6;5:175. doi: 10.3389/fphys.2014.00175. eCollection 2014. Front Physiol. 2014. PMID: 24834056 Free PMC article. Review.
-
Haemodynamic and neuroendocrine effects of tezosentan in chronic experimental pulmonary hypertension.Intensive Care Med. 2012 Jun;38(6):1050-60. doi: 10.1007/s00134-012-2484-5. Epub 2012 Feb 14. Intensive Care Med. 2012. PMID: 22349420
References
-
- Andresen JJ, Faraci FM, Heistad DD. Vasomotor responses in MnSOD-deficient mice. Am J Physiol Heart Circ Physiol 287: H1141–H1148, 2004 - PubMed
-
- Bauersachs J, Bouloumie A, Fraccarollo D, Hu K, Busse R, Ertl G. Endothelial dysfunction in chronic myocardial infarction despite increased vascular endothelial nitric oxide synthase and soluble guanylate cyclase expression: role of enhanced vascular superoxide production. Circulation 100: 292–298, 1999 - PubMed
-
- Bauersachs J, Widder JD. Endothelial dysfunction in heart failure. Pharmacol Rep 60: 119–126, 2008 - PubMed
-
- Bendall JK, Rinze R, Adlam D, Tatham AL, de Bono J, Wilson N, Volpi E, Channon KM. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ Res 100: 1016–1025, 2007 - PubMed
-
- Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol 23: 168–175, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

