TAX1BP1 and A20 inhibit antiviral signaling by targeting TBK1-IKKi kinases
- PMID: 20304918
- PMCID: PMC2865285
- DOI: 10.1074/jbc.M110.109819
TAX1BP1 and A20 inhibit antiviral signaling by targeting TBK1-IKKi kinases
Abstract
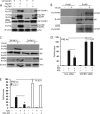
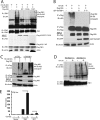
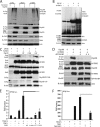
Induction of type I interferons by the transcription factor IRF3 is essential in the initiation of antiviral innate immunity. Activation of IRF3 requires C-terminal phosphorylation by the upstream kinases TBK1-IKKi, where IRF3 phosphorylation promotes dimerization, and subsequent nuclear translocation to the IFNbeta promoter. Recent studies have described the ubiquitin-editing enzyme A20 as a negative regulator of IRF3 signaling by associating with TBK1-IKKi; however, the regulatory mechanism of A20 inhibition remains unclear. Here we describe the adaptor protein, TAX1BP1, as a key regulator of A20 function in terminating signaling to IRF3. Murine embryonic fibroblasts (MEFs) deficient in TAX1BP1 displayed increased amounts of IFNbeta production upon viral challenge compared with WT MEFs. TAX1BP1 inhibited virus-mediated activation of IRF3 at the level of TBK1-IKKi. TAX1BP1 and A20 blocked antiviral signaling by disrupting Lys(63)-linked polyubiquitination of TBK1-IKKi independently of the A20 deubiquitination domain. Furthermore, TAX1BP1 was required for A20 effector function because A20 was defective for the targeting and inactivation of TBK1 and IKKi in Tax1bp1(-)(/)(-) MEFs. Additionally, we found the E3 ubiquitin ligase TRAF3 to play a critical role in promoting TBK1-IKKi ubiquitination. Collectively, our results demonstrate TBK1-IKKi to be novel substrates for A20 and further identify a novel mechanism whereby A20 and TAX1BP1 restrict antiviral signaling by disrupting a TRAF3-TBK1-IKKi signaling complex.
Figures


References
-
- Kawai T., Akira S. (2008) Ann. N.Y. Acad. Sci. 1143, 1–20 - PubMed
-
- Hiscott J., Grandvaux N., Sharma S., Tenoever B. R., Servant M. J., Lin R. (2003) Ann. N.Y. Acad. Sci. 1010, 237–248 - PubMed
-
- Medzhitov R. (2007) Nature 449, 819–826 - PubMed
-
- Honda K., Taniguchi T. (2006) Nat. Rev. Immunol. 6, 644–658 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

