Convertase inhibitory properties of Staphylococcal extracellular complement-binding protein
- PMID: 20304920
- PMCID: PMC2865291
- DOI: 10.1074/jbc.M109.091975
Convertase inhibitory properties of Staphylococcal extracellular complement-binding protein
Abstract
The human pathogen Staphylococcus aureus secretes several complement evasion molecules to combat the human immune response. Extracellular complement-binding protein (Ecb) binds to the C3d domain of C3 and thereby blocks C3 convertases of the alternative pathway and C5 convertases via all complement pathways. Inhibition of C5 convertases results in complete inhibition of C5a generation and subsequent neutrophil migration. Here, we show that binding of Ecb to the C3d domain of C3b is crucial for inhibition of C5 convertases. Ecb does not interfere with substrate binding to convertases but prevents formation of an active convertase enzyme.
Figures
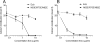



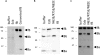

Similar articles
-
Staphylococcal complement evasion by various convertase-blocking molecules.J Exp Med. 2007 Oct 1;204(10):2461-71. doi: 10.1084/jem.20070818. Epub 2007 Sep 24. J Exp Med. 2007. PMID: 17893203 Free PMC article.
-
Staphylococcal complement inhibitor modulates phagocyte responses by dimerization of convertases.J Immunol. 2010 Jan 1;184(1):420-5. doi: 10.4049/jimmunol.0902865. Epub 2009 Nov 30. J Immunol. 2010. PMID: 19949103
-
Functional Characterization of Alternative and Classical Pathway C3/C5 Convertase Activity and Inhibition Using Purified Models.Front Immunol. 2018 Jul 23;9:1691. doi: 10.3389/fimmu.2018.01691. eCollection 2018. Front Immunol. 2018. PMID: 30083158 Free PMC article.
-
Structure/function of C5 convertases of complement.Int Immunopharmacol. 2001 Mar;1(3):415-22. doi: 10.1016/s1567-5769(00)00039-4. Int Immunopharmacol. 2001. PMID: 11367526 Review.
-
Structure and function of complement C5 convertase enzymes.Biochem Soc Trans. 2002 Nov;30(Pt 6):1006-10. doi: 10.1042/bst0301006. Biochem Soc Trans. 2002. PMID: 12440962 Review.
Cited by
-
Staphylococcus aureus surface protein SdrE binds complement regulator factor H as an immune evasion tactic.PLoS One. 2012;7(5):e38407. doi: 10.1371/journal.pone.0038407. Epub 2012 May 31. PLoS One. 2012. PMID: 22675461 Free PMC article.
-
Novel Evasion Mechanisms of the Classical Complement Pathway.J Immunol. 2016 Sep 15;197(6):2051-60. doi: 10.4049/jimmunol.1600863. J Immunol. 2016. PMID: 27591336 Free PMC article. Review.
-
Complement Inactivation Strategy of Staphylococcus aureus Using Decay-Accelerating Factor and the Response of Infected HaCaT Cells.Int J Mol Sci. 2021 Apr 13;22(8):4015. doi: 10.3390/ijms22084015. Int J Mol Sci. 2021. PMID: 33924622 Free PMC article.
-
CipA mediates complement resistance of Acinetobacter baumannii by formation of a factor I-dependent quadripartite assemblage.Front Immunol. 2022 Jul 26;13:942482. doi: 10.3389/fimmu.2022.942482. eCollection 2022. Front Immunol. 2022. PMID: 35958553 Free PMC article.
-
A genetic regulatory see-saw of biofilm and virulence in MRSA pathogenesis.Front Microbiol. 2023 Jun 22;14:1204428. doi: 10.3389/fmicb.2023.1204428. eCollection 2023. Front Microbiol. 2023. PMID: 37434702 Free PMC article. Review.
References
-
- Lowy F. D. (1998) N. Engl. J. Med. 339, 520–532 - PubMed
-
- Peacock S. J., de Silva I., Lowy F. D. (2001) Trends Microbiol. 9, 605–610 - PubMed
-
- Rooijakkers S. H., van Kessel K. P., van Strijp J. A. (2005) Trends Microbiol. 13, 596–601 - PubMed
-
- Foster T. J. (2005) Nat. Rev. Microbiol. 3, 948–958 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

