A functional role for nicotinic acid adenine dinucleotide phosphate in oxytocin-mediated contraction of uterine smooth muscle from rat
- PMID: 20304938
- PMCID: PMC2879941
- DOI: 10.1124/jpet.110.165837
A functional role for nicotinic acid adenine dinucleotide phosphate in oxytocin-mediated contraction of uterine smooth muscle from rat
Abstract
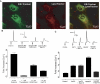
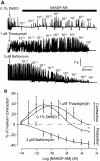
Conventionally, G protein-coupled receptors are thought to increase calcium via inositol 1,4,5-trisphosphate (InsP(3)). More recent evidence shows that an alternative second messenger, nicotinic acid adenine dinucleotide phosphate (NAADP), also has a role to play, causing researchers to question established calcium releasing pathways. With the recent development, by our group, of cell-permeant NAADP (NAADP-aceteoxymethyl ester) and a selective NAADP receptor antagonist (Ned-19; 1-(3-((4-(2-fluorophenyl)piperazin-1-yl)methyl)-4-methoxyphenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid),the ability to investigate this signaling pathway has improved. Therefore, we investigated a role for NAADP in oxytocin-mediated responses in the rat uterus. Oxytocin- and NAADP-mediated effects were investigated by using contractile measurements of whole uterine strips from rat in organ baths. Responses were correlated to calcium release in cultured rat uterine smooth muscle cells measured by fluorescence microscopy. Inhibition of both oxytocin-induced contraction and calcium release by the traditional NAADP-signaling disrupter bafilomycin and the NAADP receptor antagonist Ned-19 clearly demonstrated a role for NAADP in oxytocin-induced signaling. A cell-permeant form of NAADP was able to produce both uterine contractions and calcium release. This response was unaffected by depletion of sarcoplasmic reticulum stores with thapsigargin, but was abolished by both bafilomycin and Ned-19. Crucially, oxytocin stimulated an increase in NAADP in rat uterine tissue. The present study demonstrates directly that NAADP signaling plays a role in rat uterine contractions. Moreover, investigation of this signaling pathway highlights yet another component of oxytocin-mediated signaling, stressing the need to consider the action of new components as they are discovered, even in signaling pathways that are thought to be well established.
Figures


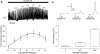

References
-
- Aarhus R, Dickey DM, Graeff RM, Gee KR, Walseth TF, Lee HC. (1996) Activation and inactivation of Ca2+ release by NAADP. J Biol Chem 271:8513–8516 - PubMed
-
- Barata H, Thompson M, Zielinska W, Han YS, Mantilla CB, Prakash YS, Feitoza S, Sieck G, Chini EN. (2004) The role of cyclic-ADP-ribose-signaling pathway in oxytocin-induced Ca2+ transients in human myometrium cells. Endocrinology 145:881–889 - PubMed
-
- Billington RA, Genazzani AA. (2000) Characterization of NAADP(+) binding in sea urchin eggs. Biochem Biophys Res Commun 276:112–116 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

