Nitro-oleic acid inhibits firing and activates TRPV1- and TRPA1-mediated inward currents in dorsal root ganglion neurons from adult male rats
- PMID: 20304940
- PMCID: PMC2879937
- DOI: 10.1124/jpet.109.163154
Nitro-oleic acid inhibits firing and activates TRPV1- and TRPA1-mediated inward currents in dorsal root ganglion neurons from adult male rats
Abstract
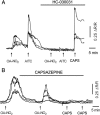
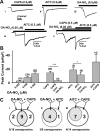
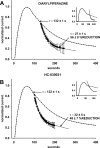
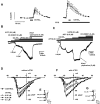
Nitro-oleic acid (OA-NO(2)), an electrophilic fatty acid by-product of nitric oxide and nitrite reactions, is present in normal and inflamed mammalian tissues at up to micromolar concentrations and exhibits anti-inflammatory signaling actions. The effects of OA-NO(2) on cultured dorsal root ganglion (DRG) neurons were examined using fura-2 Ca(2+) imaging and patch clamping. OA-NO(2) (3.5-35 microM) elicited Ca(2+) transients in 20 to 40% of DRG neurons, the majority (60-80%) of which also responded to allyl isothiocyanate (AITC; 1-50 microM), a TRPA1 agonist, and to capsaicin (CAPS; 0.5 microM), a TRPV1 agonist. The OA-NO(2)-evoked Ca(2+) transients were reduced by the TRPA1 antagonist 2-(1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-7H-purin-7-yl)-N-(4-isopropylphenyl) acetamide (HC-030031; 5-50 microM) and the TRPV1 antagonist capsazepine (10 microM). Patch-clamp recording revealed that OA-NO(2) depolarized and induced inward currents in 62% of neurons. The effects of OA-NO(2) were elicited by concentrations >or=5 nM and were blocked by 10 mM dithiothreitol. Concentrations of OA-NO(2) >or=5 nM reduced action potential (AP) overshoot, increased AP duration, inhibited firing induced by depolarizing current pulses, and inhibited Na(+) currents. The effects of OA-NO(2) were not prevented or reversed by the NO-scavenger carboxy-2-phenyl-4,4,5,5-tetramethylimidazolineoxyl-1-oxyl-3-oxide. A large percentage (46-57%) of OA-NO(2)-responsive neurons also responded to CAPS (0.5 microM) or AITC (0.5 microM). OA-NO(2) currents were reduced by TRPV1 (diarylpiperazine; 5 microM) or TRPA1 (HC-030031; 5 microM) antagonists. These data reveal that endogenous OA-NO(2) generated at sites of inflammation may initially activate transient receptor potential channels on nociceptive afferent nerves, contributing to the initiation of afferent nerve activity, and later suppresses afferent firing.
Figures





Similar articles
-
Nitro-oleic acid desensitizes TRPA1 and TRPV1 agonist responses in adult rat DRG neurons.Exp Neurol. 2014 Jan;251:12-21. doi: 10.1016/j.expneurol.2013.10.020. Epub 2013 Nov 8. Exp Neurol. 2014. PMID: 24212047 Free PMC article.
-
Activation of TRPC channels contributes to OA-NO2-induced responses in guinea-pig dorsal root ganglion neurons.J Physiol. 2014 Oct 1;592(19):4297-312. doi: 10.1113/jphysiol.2014.271783. Epub 2014 Aug 15. J Physiol. 2014. PMID: 25128576 Free PMC article.
-
Activation characteristics of transient receptor potential ankyrin 1 and its role in nociception.Am J Physiol Cell Physiol. 2011 Sep;301(3):C587-600. doi: 10.1152/ajpcell.00465.2010. Epub 2011 Jun 8. Am J Physiol Cell Physiol. 2011. PMID: 21653898 Free PMC article.
-
Functional TRP and ASIC-like channels in cultured urothelial cells from the rat.Am J Physiol Renal Physiol. 2009 Apr;296(4):F892-901. doi: 10.1152/ajprenal.90718.2008. Epub 2009 Jan 21. Am J Physiol Renal Physiol. 2009. PMID: 19158342 Free PMC article.
-
The effect of the electrophilic fatty acid nitro-oleic acid on TRP channel function in sensory neurons.Nitric Oxide. 2018 Mar 22:S1089-8603(17)30289-6. doi: 10.1016/j.niox.2018.03.015. Online ahead of print. Nitric Oxide. 2018. PMID: 29578059 Free PMC article. Review.
Cited by
-
Post-Translational Modification of Proteins Mediated by Nitro-Fatty Acids in Plants: Nitroalkylation.Plants (Basel). 2019 Mar 29;8(4):82. doi: 10.3390/plants8040082. Plants (Basel). 2019. PMID: 30934982 Free PMC article. Review.
-
The transient receptor potential channel TRPA1: from gene to pathophysiology.Pflugers Arch. 2012 Nov;464(5):425-58. doi: 10.1007/s00424-012-1158-z. Epub 2012 Sep 22. Pflugers Arch. 2012. PMID: 23001121 Review.
-
Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids.Annu Rev Physiol. 2014;76:79-105. doi: 10.1146/annurev-physiol-021113-170341. Epub 2013 Oct 16. Annu Rev Physiol. 2014. PMID: 24161076 Free PMC article. Review.
-
Discovery of bioactive nitrated lipids and nitro-lipid-protein adducts using mass spectrometry-based approaches.Redox Biol. 2019 May;23:101106. doi: 10.1016/j.redox.2019.101106. Epub 2019 Jan 14. Redox Biol. 2019. PMID: 30718106 Free PMC article. Review.
-
Inflammation-induced increase in nicotinic acetylcholine receptor current in cutaneous nociceptive DRG neurons from the adult rat.Neuroscience. 2015 Jan 22;284:483-499. doi: 10.1016/j.neuroscience.2014.10.018. Epub 2014 Oct 18. Neuroscience. 2015. PMID: 25453771 Free PMC article.
References
-
- Baker PR, Lin Y, Schopfer FJ, Woodcock SR, Groeger AL, Batthyany C, Sweeney S, Long MH, Iles KE, Baker LM, et al. (2005) Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem 280:42464–42475 - PMC - PubMed
-
- Dang K, Bielefeldt K, Gebhart GF. (2004) Gastric ulcers reduce A-type potassium currents in rat gastric sensory ganglion neurons. Am J Physiol Gastrointest Liver Physiol 286:G573–G579 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

