Developing an articular cartilage decellularization process toward facet joint cartilage replacement
- PMID: 20305493
- PMCID: PMC3164496
- DOI: 10.1227/01.NEU.0000367616.49291.9F
Developing an articular cartilage decellularization process toward facet joint cartilage replacement
Abstract
Objective: The facet joint has been identified as a significant source of morbidity in lower back pain. In general, treatments have focused on reducing the pain associated with facet joint osteoarthritis, and no treatments have targeted the development of a replacement tissue for arthritic facet articular cartilage. Therefore, the objective of this study was to develop a nonimmunogenic decellularized articular cartilage replacement tissue while maintaining functional properties similar to native facet cartilage tissue.
Methods: In vitro testing was performed on bovine articular cartilage explants. The effects of 2% sodium dodecyl sulfate (SDS), a detergent used for cell and nuclear membrane solubilization, on cartilage cellularity, biochemical, and biomechanical properties, were examined. Compressive biomechanical properties were determined using creep indentation, and the tensile biomechanical properties were obtained with uniaxial tensile testing. Biochemical assessment involved determination of the DNA content, glycosaminoglycan (GAG) content, and collagen content. Histological examination included hematoxylin and eosin staining for tissue cellularity, as well as staining for collagen and GAG.
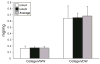
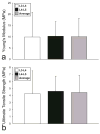
Results: Treatment with 2% SDS for 2 hours maintained the compressive and tensile biomechanical properties, as well as the GAG and collagen content while resulting in a decrease in cell nuclei and a 4% decrease in DNA content. Additionally, treatment for 8 hours resulted in complete histological decellularization and a 40% decrease in DNA content while maintaining collagen content and tensile properties. However, a significant decrease in compressive properties and GAG content was observed. Similar results were observed with 4 hours of treatment, although the decrease in DNA content was not as great as with 8 hours of treatment.
Conclusion: Treatment with 2% SDS for 8 hours resulted in complete histological decellularization with decreased mechanical properties, whereas treatment for 2 hours maintained mechanical properties, but had a minimal effect on DNA content. Therefore, future studies must be performed to optimize a treatment for decellularization while maintaining mechanical properties close to those of facet joint cartilage. This study served as a step in creating a decellularized articular cartilage replacement tissue that could be used as a treatment for facet cartilage osteoarthritis.
Figures





Similar articles
-
Extraction techniques for the decellularization of tissue engineered articular cartilage constructs.Biomaterials. 2009 Aug;30(22):3749-56. doi: 10.1016/j.biomaterials.2009.03.050. Epub 2009 Apr 23. Biomaterials. 2009. PMID: 19395023 Free PMC article.
-
Biomechanical, biochemical, and histological characterization of canine lumbar facet joint cartilage.J Neurosurg Spine. 2009 Jun;10(6):623-8. doi: 10.3171/2009.2.SPINE08818. J Neurosurg Spine. 2009. PMID: 19558298
-
Biochemical and biomechanical characterisation of equine cervical facet joint cartilage.Equine Vet J. 2018 Nov;50(6):800-808. doi: 10.1111/evj.12845. Epub 2018 May 17. Equine Vet J. 2018. PMID: 29658148
-
Decellularization of porcine articular cartilage explants and their subsequent repopulation with human chondroprogenitor cells.J Mech Behav Biomed Mater. 2015 Mar;55:21-31. doi: 10.1016/j.jmbbm.2015.10.002. Epub 2015 Oct 22. J Mech Behav Biomed Mater. 2015. PMID: 26521085
-
A comparison study of different decellularization treatments on bovine articular cartilage.J Tissue Eng Regen Med. 2019 Oct;13(10):1861-1871. doi: 10.1002/term.2936. Epub 2019 Aug 27. J Tissue Eng Regen Med. 2019. PMID: 31314950
Cited by
-
A novel 3D histotypic cartilage construct engineered by supercritical carbon dioxide decellularized porcine nasal cartilage graft and chondrocytes exhibited chondrogenic capability in vitro.Int J Med Sci. 2021 Mar 25;18(10):2217-2227. doi: 10.7150/ijms.56342. eCollection 2021. Int J Med Sci. 2021. PMID: 33859530 Free PMC article.
-
Decellularized Extracellular Matrix as a Potent Natural Biomaterial for Regenerative Medicine.Adv Exp Med Biol. 2021;1341:27-43. doi: 10.1007/5584_2020_504. Adv Exp Med Biol. 2021. PMID: 32166633
-
Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds.Int J Mol Sci. 2020 Jul 30;21(15):5447. doi: 10.3390/ijms21155447. Int J Mol Sci. 2020. PMID: 32751654 Free PMC article. Review.
-
Rapid porcine corneal decellularization through the use of sodium N-lauroyl glutamate and supernuclease.J Tissue Eng. 2019 Sep 11;10:2041731419875876. doi: 10.1177/2041731419875876. eCollection 2019 Jan-Dec. J Tissue Eng. 2019. PMID: 31588337 Free PMC article.
-
Yucatan Minipig Knee Meniscus Regional Biomechanics and Biochemical Structure Support its Suitability as a Large Animal Model for Translational Research.Front Bioeng Biotechnol. 2022 Feb 21;10:844416. doi: 10.3389/fbioe.2022.844416. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35265605 Free PMC article.
References
-
- Athanasiou KA, Agarwal A, Dzida FJ. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. J Orthop Res. 1994;12:340–349. - PubMed
-
- Athanasiou KA, Agarwal A, Muffoletto A, Dzida FJ, Constantinides G, Clem M. Biomechanical properties of hip cartilage in experimental animal models. Clin Orthop Relat Res. 1995:254–266. - PubMed
-
- Athanasiou KA, Rosenwasser MP, Buckwalter JA, Malinin TI, Mow VC. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J Orthop Res. 1991;9:330–340. - PubMed
-
- Aufderheide AC, Athanasiou KA. Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs. Tissue Eng. 2007;13:2195–2205. - PubMed
-
- Boswell MV, Colson JD, Sehgal N, Dunbar EE, Epter R. A systematic review of therapeutic facet joint interventions in chronic spinal pain. Pain Physician. 2007;10:229–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

