The brain tumor window model: a combined cranial window and implanted glioma model for evaluating intraoperative contrast agents
- PMID: 20305495
- PMCID: PMC3970731
- DOI: 10.1227/01.NEU.0000367631.02903.50
The brain tumor window model: a combined cranial window and implanted glioma model for evaluating intraoperative contrast agents
Abstract
Objective: Optical contrast agents for brain tumor delineation have been previously evaluated in ex vivo specimens from animals with implanted gliomas and may not reflect the true visual parameters encountered during surgery. This study describes a novel model system designed to evaluate optical contrast agents for tumor delineation in vivo.
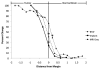
Methods: Biparietal craniectomies were performed on 8-week-old Sprague-Dawley rats. 9L glioma cells were injected intraparenchymally. A cover slip was bonded to the cranial defect with cyanoacrylate glue. When the tumor radius reached 1 mm, Coomassie Blue was administered intravenously while the appearance of the cortical surface was recorded. Computerized image analysis of the red/green/blue color components was used to quantify visible differences between tumor and nonneoplastic tissue and to compare delineation in the brain tumor window (BTW) model with the conventional 9L glioma model.
Results: The tumor margin in the BTW model was poorly defined before contrast administration but readily apparent after contrast administration. Based on red component intensity, tumor delineation improved 4-fold at 50 minutes after contrast administration in the BTW model (P < .002). The conventional 9L glioma model overestimated the degree of delineation compared with the BTW model at the same dose of Coomassie Blue (P < .03).
Conclusion: Window placement overlying an implanted glioma is technically possible and well tolerated in the rat. The BTW model is a valid system for evaluating optical contrast agents designed to delineate brain tumor margins. To our knowledge, we have described the first in vivo model system for evaluating optical contrast agents for tumor delineation.
Figures








Similar articles
-
A technical description of the brain tumor window model: an in vivo model for the evaluation of intraoperative contrast agents.Acta Neurochir Suppl. 2011;109:259-63. doi: 10.1007/978-3-211-99651-5_41. Acta Neurochir Suppl. 2011. PMID: 20960353 Free PMC article.
-
Gliomas: Motexafin Gadolinium-enhanced Molecular MR Imaging and Optical Imaging for Potential Intraoperative Delineation of Tumor Margins.Radiology. 2016 May;279(2):400-9. doi: 10.1148/radiol.2015150895. Epub 2015 Nov 24. Radiology. 2016. PMID: 26599802 Free PMC article.
-
Molecular susceptibility weighted imaging of the glioma rim in a mouse model.J Neurosci Methods. 2014 Apr 15;226:132-138. doi: 10.1016/j.jneumeth.2014.01.034. Epub 2014 Feb 10. J Neurosci Methods. 2014. PMID: 24525326
-
Surface-Enhanced Resonance Raman Scattering-Guided Brain Tumor Surgery Showing Prognostic Benefit in Rat Models.ACS Appl Mater Interfaces. 2019 May 1;11(17):15241-15250. doi: 10.1021/acsami.9b00227. Epub 2019 Apr 17. ACS Appl Mater Interfaces. 2019. PMID: 30896915
-
Rat brain tumor models in experimental neuro-oncology: the 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas.J Neurooncol. 1998 Jan;36(1):91-102. doi: 10.1023/a:1005805203044. J Neurooncol. 1998. PMID: 9525831 Review.
Cited by
-
Through the looking glass: A review of cranial window technology for optical access to the brain.J Neurosci Methods. 2021 Apr 15;354:109100. doi: 10.1016/j.jneumeth.2021.109100. Epub 2021 Feb 15. J Neurosci Methods. 2021. PMID: 33600850 Free PMC article. Review.
-
Disposable ultrasound-sensing chronic cranial window by soft nanoimprinting lithography.Nat Commun. 2019 Sep 19;10(1):4277. doi: 10.1038/s41467-019-12178-6. Nat Commun. 2019. PMID: 31537800 Free PMC article.
-
Guiding brain tumor resection using surface-enhanced Raman scattering nanoparticles and a hand-held Raman scanner.ACS Nano. 2014 Oct 28;8(10):9755-66. doi: 10.1021/nn503948b. Epub 2014 Aug 22. ACS Nano. 2014. PMID: 25093240 Free PMC article.
-
Design and Synthesis of SERS Materials for In Vivo Molecular Imaging and Biosensing.Adv Sci (Weinh). 2023 Mar;10(8):e2202051. doi: 10.1002/advs.202202051. Epub 2023 Jan 22. Adv Sci (Weinh). 2023. PMID: 36683237 Free PMC article. Review.
-
The translocator protein ligand [¹⁸F]DPA-714 images glioma and activated microglia in vivo.Eur J Nucl Med Mol Imaging. 2012 May;39(5):811-23. doi: 10.1007/s00259-011-2041-4. Epub 2012 Jan 21. Eur J Nucl Med Mol Imaging. 2012. PMID: 22270507 Free PMC article.
References
-
- Central Brain Tumor Registry of the United States. Primary Brain Tumors in the United States Statistical Report. 2007 http://www.cbtrus.org/reports//2007-2008/2007report.pdf. Updated Last Updated Date.
-
- Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008 Apr;62(4):753–764. discussion 264-756. - PubMed
-
- Britz G, Ghatan S, Spence AM, Berger MS. Intracarotid RMP-7 enhanced indocyanine green staining of tumors in a rat glioma model. Journal of Neurooncology. 2002;56:227–232. - PubMed
-
- Hansen D, Spence AM, Carski T, Berger M. Indocyanine green (ICG) staining and demarcation of tumor margins in a rat glioma model. Surgical Neurology. 1993;40:451–456. - PubMed
-
- Moore G. Fluorescein as an agent in the differentiation of normal and malignant tissues. Science. 1947;106:130–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

