Monitoring neural activity with bioluminescence during natural behavior
- PMID: 20305645
- PMCID: PMC2846983
- DOI: 10.1038/nn.2518
Monitoring neural activity with bioluminescence during natural behavior
Abstract
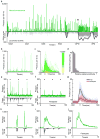
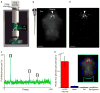
Existing techniques for monitoring neural activity in awake, freely behaving vertebrates are invasive and difficult to target to genetically identified neurons. We used bioluminescence to non-invasively monitor the activity of genetically specified neurons in freely behaving zebrafish. Transgenic fish with the Ca(2+)-sensitive photoprotein green fluorescent protein (GFP)-Aequorin in most neurons generated large and fast bioluminescent signals that were related to neural activity, neuroluminescence, which could be recorded continuously for many days. To test the limits of this technique, we specifically targeted GFP-Aequorin to the hypocretin-positive neurons of the hypothalamus. We found that neuroluminescence generated by this group of approximately 20 neurons was associated with periods of increased locomotor activity and identified two classes of neural activity corresponding to distinct swim latencies. Our neuroluminescence assay can report, with high temporal resolution and sensitivity, the activity of small subsets of neurons during unrestrained behavior.
Figures




Comment in
-
Neurons light the way.Nat Methods. 2010 May;7(5):346. doi: 10.1038/nmeth0510-346. Nat Methods. 2010. PMID: 20440882 No abstract available.
References
-
- Kralik JD, et al. Techniques for long-term multisite neuronal ensemble recordings in behaving animals. Methods. 2001;25:121–150. - PubMed
-
- Miller EK, Wilson MA. All my circuits: using multiple electrodes to understand functioning neural networks. Neuron. 2008;60:483–488. - PubMed
-
- Brustein E, Marandi N, Kovalchuk Y, Drapeau P, Konnerth A. “In vivo” monitoring of neuronal network activity in zebrafish by two-photon Ca(2+) imaging. Pflugers Arch. 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

