Cytotoxic effects of G(M1) ganglioside and amyloid β-peptide on mouse embryonic neural stem cells
- PMID: 20305711
- PMCID: PMC2838405
- DOI: 10.1042/AN20090063
Cytotoxic effects of G(M1) ganglioside and amyloid β-peptide on mouse embryonic neural stem cells
Abstract
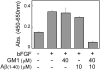
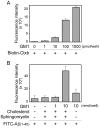
AD (Alzheimer's disease) is a neurodegenerative disease and the most common form of dementia. One of the pathological hallmarks of AD is the aggregation of extracellular Aβs (amyloid β-peptides) in senile plaques in the brain. The process could be initiated by seeding provided by an interaction between G(M1) ganglioside and Aβs. Several reports have documented the bifunctional roles of Aβs in NSCs (neural stem cells), but the precise effects of G(M1) and Aβ on NSCs have not yet been clarified. We evaluated the effect of G(M1) and Aβ-(1-40) on mouse NECs (neuroepithelial cells), which are known to be rich in NSCs. No change of cell number was detected in NECs cultured in the presence of either G(M1) or Aβ-(1-40). On the contrary, a decreased number of NECs were cultured in the presence of a combination of G(M1) and Aβ-(1-40). The exogenously added G(M1) and Aβ-(1-40) were confirmed to incorporate into NECs. The Ras-MAPK (mitogen-activated protein kinase) pathway, important for cell proliferation, was intact in NECs simultaneously treated with G(M1) and Aβ-(1-40), but caspase 3 was activated. NECs treated with G(M1) and Aβ-(1-40) were positive in the TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling) assay, an indicator of cell death. It was found that G(M1) and Aβ-(1-40) interacted in the presence of cholesterol and sphingomyelin, components of cell surface microdomains. The cytotoxic effect was found also in NSCs prepared via neurospheres. These results indicate that Aβ-(1-40) and G(M1) co-operatively exert a cytotoxic effect on NSCs, likely via incorporation into NEC membranes, where they form a complex for the activation of cell death signalling.
Keywords: AD, Alzheimer’s disease; Alzheimer’s disease (AD); Aβ, amyloid β-peptide; CCD, charge-coupled device; DMEM, Dulbecco’s modified Eagle’s medium; ERK, extracellular-signal-regulated kinase; FITC-Aβ-(1–40), FITC-conjugated Aβ-(1–40); GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GM1 ganglioside; IACUC, Institutional Animal Care and Use Committee; IL, interleukin; MAP2, microtubule-associated protein 2; MAPK, mitogen-activated protein kinase; N2-DMEM/F12, N2-supplemented DMEM/Ham’s Nutrient Mixture F12; NEC, neuroepithelial cell; NSC, neural stem cell; RT–PCR, reverse transcription–PCR; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling; amyloid β-peptide (Aβ); apoptosis; bFGF, basic fibroblast growth factor; biotin-Ctxb, biotin-conjugated cholera toxin B subunit; glycosphingolipid; neural stem cell.
Figures







Similar articles
-
Ganglioside-Dependent Neural Stem Cell Proliferation in Alzheimer's Disease Model Mice.ASN Neuro. 2015 Dec 23;7(6):1759091415618916. doi: 10.1177/1759091415618916. Print 2015 Nov-Dec. ASN Neuro. 2015. PMID: 26699276 Free PMC article.
-
Effects of amyloid β-peptides and gangliosides on mouse neural stem cells.Neurochem Res. 2013 Oct;38(10):2019-27. doi: 10.1007/s11064-013-1108-y. Epub 2013 Jul 14. Neurochem Res. 2013. PMID: 23851714 Free PMC article.
-
Activation of the endoplasmic reticulum stress response by the amyloid-beta 1-40 peptide in brain endothelial cells.Biochim Biophys Acta. 2013 Dec;1832(12):2191-203. doi: 10.1016/j.bbadis.2013.08.007. Epub 2013 Aug 28. Biochim Biophys Acta. 2013. PMID: 23994613
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Aβ-ganglioside interactions in the pathogenesis of Alzheimer's disease.Biochim Biophys Acta Biomembr. 2020 Aug 1;1862(8):183233. doi: 10.1016/j.bbamem.2020.183233. Epub 2020 Mar 3. Biochim Biophys Acta Biomembr. 2020. PMID: 32142821 Review.
Cited by
-
Ganglioside GM1 and the Central Nervous System.Int J Mol Sci. 2023 May 31;24(11):9558. doi: 10.3390/ijms24119558. Int J Mol Sci. 2023. PMID: 37298512 Free PMC article. Review.
-
Sialometabolism in Brain Health and Alzheimer's Disease.Front Neurosci. 2021 Mar 30;15:648617. doi: 10.3389/fnins.2021.648617. eCollection 2021. Front Neurosci. 2021. PMID: 33867926 Free PMC article. Review.
-
β-Amyloid (1-40) peptide interactions with supported phospholipid membranes: a single-molecule study.Biophys J. 2012 Oct 3;103(7):1500-9. doi: 10.1016/j.bpj.2012.08.051. Epub 2012 Oct 2. Biophys J. 2012. PMID: 23062342 Free PMC article.
-
Ganglioside-Dependent Neural Stem Cell Proliferation in Alzheimer's Disease Model Mice.ASN Neuro. 2015 Dec 23;7(6):1759091415618916. doi: 10.1177/1759091415618916. Print 2015 Nov-Dec. ASN Neuro. 2015. PMID: 26699276 Free PMC article.
-
Different amyloid β42 preparations induce different cell death pathways in the model of SH-SY5Y neuroblastoma cells.Cell Mol Biol Lett. 2024 Nov 17;29(1):143. doi: 10.1186/s11658-024-00657-8. Cell Mol Biol Lett. 2024. PMID: 39551742 Free PMC article.
References
-
- Ariga T, Yu RK. GM1 inhibits amyloid β-protein-induced cytokine release. Neurochem Res. 1999;24:219–226. - PubMed
-
- Ariga T, Kiso M, Hasegawa A, Miyatake T. Gangliosides inhibit the release of interleukin-1β in amyloid β-protein-treated human monocytic cells. J Mol Neurosci. 2001a;17:371–377. - PubMed
-
- Ariga T, Kobayashi K, Hasegawa A, Kiso M, Ishida H, Miyatake T. Characterization of high-affinity binding between gangliosides and amyloid β-protein. Arch Biochem Biophys. 2001b;388:225–230. - PubMed
-
- Bernardo A, Harrison FE, McCord M, Zhao J, Bruchey A, Davies SS, Jackson Roberts L, II, Mathews PM, Matsuoka Y, Ariga T, Yu RK, Thompson R, McDonald MP. Elimination of GD3 synthase improves memory and reduces amyloid-β plaque load in transgenic mice. Neurobiol Aging. 2009;30:1777–1791. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
